Acinetobacter baumannii secretes cytotoxic outer membrane protein A via outer membrane vesicles
- PMID: 21386968
- PMCID: PMC3046175
- DOI: 10.1371/journal.pone.0017027
Acinetobacter baumannii secretes cytotoxic outer membrane protein A via outer membrane vesicles
Abstract
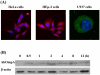
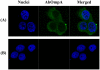
Acinetobacter baumannii is an important nosocomial pathogen that causes a high morbidity and mortality rate in infected patients, but pathogenic mechanisms of this microorganism regarding the secretion and delivery of virulence factors to host cells have not been characterized. Gram-negative bacteria naturally secrete outer membrane vesicles (OMVs) that play a role in the delivery of virulence factors to host cells. A. baumannii has been shown to secrete OMVs when cultured in vitro, but the role of OMVs in A. baumannii pathogenesis is not well elucidated. In the present study, we evaluated the secretion and delivery of virulence factors of A. baumannii to host cells via the OMVs and assessed the cytotoxic activity of outer membrane protein A (AbOmpA) packaged in the OMVs. A. baumannii ATCC 19606(T) secreted OMVs during in vivo infection as well as in vitro cultures. Potential virulence factors, including AbOmpA and tissue-degrading enzymes, were associated with A. baumannii OMVs. A. baumannii OMVs interacted with lipid rafts in the plasma membranes and then delivered virulence factors to host cells. The OMVs from A. baumannii ATCC 19606(T) induced apoptosis of host cells, whereas this effect was not detected in the OMVs from the ΔompA mutant, thereby reflecting AbOmpA-dependent host cell death. The N-terminal region of AbOmpA(22-170) was responsible for host cell death. In conclusion, the OMV-mediated delivery of virulence factors to host cells may well contribute to pathogenesis during A. baumannii infection.
Conflict of interest statement
Figures

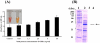
References
-
- Dijkshoorn L, Nemec A, Seifert H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol. 2007;5:939–951. - PubMed
-
- Gordon MC, Wareham DW. Multidrug-resistant Acinetobacter baumannii: mechanisms of virulence and resistance. Int J Antimicrob Agents. 2010;35:219–226. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

