Subgroup analysis of large trials can guide further research: a case study of vitamin E and pneumonia
- PMID: 21386974
- PMCID: PMC3046185
- DOI: 10.2147/CLEP.S16114
Subgroup analysis of large trials can guide further research: a case study of vitamin E and pneumonia
Abstract
Background: Biology is complex and the effects of many interventions may vary between population groups. Subgroup analysis can give estimates for specific populations, but trials are usually too small for such analyses.
Purpose: To test whether the effect of vitamin E on pneumonia risk is uniform over subgroups defined by smoking and exercise.
Methods: The Alpha-Tocopherol Beta-Carotene Cancer Prevention Study examined the effects of vitamin E (50 mg per day) and β-carotene (20 mg per day) on lung cancer in 29,133 male smokers aged 50-69 years using a 2 × 2 factorial design. The trial was conducted among the general community in Finland during 1985-1993; the intervention lasted for 6.0 years (median). In the present study, we tested the uniformity of vitamin E effect on the risk of hospital-treated pneumonia (898 cases) by adding a dummy variable to allow each subgroup its own vitamin E effect in a Cox model covering all participants.
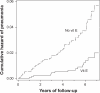
Results: Vitamin E effect was not uniform over eight subgroups defined by baseline smoking (5-19 vs ≥20 cigarettes per day), age of smoking initiation (≤20 vs ≥21 years), and exercise during leisure time (yes vs no). Vitamin E decreased pneumonia risk by 69% (95% CI: 43% to 83%) among participants who had the least exposure to smoking and exercised during leisure time. Vitamin E increased pneumonia risk by 79% (95% CI: 27% to 150%) among those who had the highest exposure to smoking and did not exercise.
Limitations: Although the evidence of heterogeneity is strong, it is not evident to what extent the estimates of effect or the limits between the subgroups can be extrapolated to other populations.
Conclusion: Subgroup analysis of large trials should be encouraged, though caution is needed in the interpretation of findings. The role of vitamin E in susceptibility to pneumonia in physically active nonsmokers warrants further study.
Trial registration: ClinicalTrials.gov NCT00342992.
Keywords: leisure time exercise; pneumonia; smoking; subgroup analysis; vitamin E; α-tocopherol; β-carotene.
Figures
References
-
- Assmann SF, Pocock SJ, Enos LE, Kasten LE. Subgroup analysis and other (mis)uses of baseline data in clinical trials. Lancet. 2000;355:1064–1069. - PubMed
-
- Altman DG. Within trial variation–a false trail? J Clin Epidemiol. 1998;51:301–303. - PubMed
-
- Brookes ST, Whitely E, Egger M, Smith GD, Mulheran PA, Peters TJ. Subgroup analyses in randomized trials: risks of subgroup-specific analyses; power and sample size for the interaction test. J Clin Epidemiol. 2004;57:229–236. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

