Lipid pathway alterations in Parkinson's disease primary visual cortex
- PMID: 21387008
- PMCID: PMC3046155
- DOI: 10.1371/journal.pone.0017299
Lipid pathway alterations in Parkinson's disease primary visual cortex
Abstract
Background: We present a lipidomics analysis of human Parkinson's disease tissues. We have focused on the primary visual cortex, a region that is devoid of pathological changes and Lewy bodies; and two additional regions, the amygdala and anterior cingulate cortex which contain Lewy bodies at different disease stages but do not have as severe degeneration as the substantia nigra.
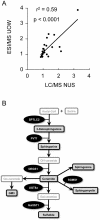
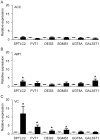
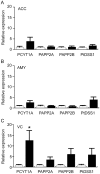
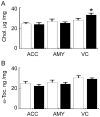
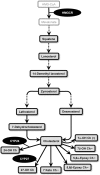
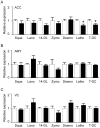
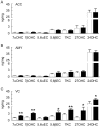
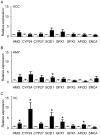
Methodology/principal findings: Using liquid chromatography mass spectrometry lipidomics techniques for an initial screen of 200 lipid species, significant changes in 79 sphingolipid, glycerophospholipid and cholesterol species were detected in the visual cortex of Parkinson's disease patients (n = 10) compared to controls (n = 10) as assessed by two-sided unpaired t-test (p-value <0.05). False discovery rate analysis confirmed that 73 of these 79 lipid species were significantly changed in the visual cortex (q-value <0.05). By contrast, changes in 17 and 12 lipid species were identified in the Parkinson's disease amygdala and anterior cingulate cortex, respectively, compared to controls; none of which remained significant after false discovery rate analysis. Using gas chromatography mass spectrometry techniques, 6 out of 7 oxysterols analysed from both non-enzymatic and enzymatic pathways were also selectively increased in the Parkinson's disease visual cortex. Many of these changes in visual cortex lipids were correlated with relevant changes in the expression of genes involved in lipid metabolism and an oxidative stress response as determined by quantitative polymerase chain reaction techniques.
Conclusions/significance: The data indicate that changes in lipid metabolism occur in the Parkinson's disease visual cortex in the absence of obvious pathology. This suggests that normalization of lipid metabolism and/or oxidative stress status in the visual cortex may represent a novel route for treatment of non-motor symptoms, such as visual hallucinations, that are experienced by a majority of Parkinson's disease patients.
Conflict of interest statement
Figures

References
-
- Fahn S. Description of Parkinson's disease as a clinical syndrome. Ann N Y Acad Sci. 2003;991:1–14. - PubMed
-
- Obeso JA, Rodriguez-Oroz MC, Goetz CG, Marin C, Kordower JH, et al. Missing pieces in the Parkinson's disease puzzle. Nat Med. 2010;16:653–661. - PubMed
-
- Dickson DW, Braak H, Duda JE, Duyckaerts C, Gasser T, et al. Neuropathological assessment of Parkinson's disease: refining the diagnostic criteria. Lancet Neurol. 2009;8:1150–1157. - PubMed
-
- Gai WP, Yuan HX, Li XQ, Power JT, Blumbergs PC, et al. In situ and in vitro study of colocalization and segregation of alpha-synuclein, ubiquitin, and lipids in Lewy bodies. Exp Neurol. 2000;166:324–333. - PubMed
-
- Halliday GM, Ophof A, Broe M, Jensen PH, Kettle E, et al. Alpha-synuclein redistributes to neuromelanin lipid in the substantia nigra early in Parkinson's disease. Brain. 2005;128:2654–2664. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

