Synthetic mimics of antimicrobial peptides from triaryl scaffolds
- PMID: 21388190
- PMCID: PMC3072574
- DOI: 10.1021/jm101410t
Synthetic mimics of antimicrobial peptides from triaryl scaffolds
Abstract
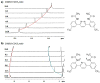
In this report, we describe the synthesis of a new series of small amphiphilic aromatic compounds that mimic the essential properties of cationic antimicrobial peptides using Suzuki-Miyaura coupling. The new design allowed the easy tuning of the conformational restriction, controlled by introduction of intramolecular hydrogen bonds, and the overall hydrophobicity by modifications to the central ring and the side chains. This approach allowed us to better understand the influence of these features on the antimicrobial activity and selectivity. We found that the overall hydrophobicity had a more significant impact on antimicrobial and hemolytic activity than the conformational stiffness. A novel compound was discovered which has MICs of 0.78 μg/mL against S. Aureus and 6.25 μg/mL against E. Coli, similar to the well-known antimicrobial peptide, MSI-78.
Figures

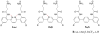




Similar articles
-
Antimicrobial benzodiazepine-based short cationic peptidomimetics.J Pept Sci. 2015 Jun;21(6):512-9. doi: 10.1002/psc.2771. Epub 2015 Mar 25. J Pept Sci. 2015. PMID: 25807936
-
Peptide/Peptoid Hybrid Oligomers: The Influence of Hydrophobicity and Relative Side-Chain Length on Antibacterial Activity and Cell Selectivity.Molecules. 2019 Dec 4;24(24):4429. doi: 10.3390/molecules24244429. Molecules. 2019. PMID: 31817108 Free PMC article.
-
De Novo Design of Flavonoid-Based Mimetics of Cationic Antimicrobial Peptides: Discovery, Development, and Applications.Acc Chem Res. 2021 Jan 5;54(1):104-119. doi: 10.1021/acs.accounts.0c00550. Epub 2020 Dec 21. Acc Chem Res. 2021. PMID: 33346639
-
Cationic Amphiphilic Peptides: Synthetic Antimicrobial Agents Inspired by Nature.ChemMedChem. 2020 Oct 19;15(20):1887-1896. doi: 10.1002/cmdc.202000301. Epub 2020 Sep 8. ChemMedChem. 2020. PMID: 32767819 Review.
-
Recent Advances in Amphipathic Peptidomimetics as Antimicrobial Agents to Combat Drug Resistance.Molecules. 2024 May 24;29(11):2492. doi: 10.3390/molecules29112492. Molecules. 2024. PMID: 38893366 Free PMC article. Review.
Cited by
-
Detection of bacteria using antimicrobial polymer derived via ring-opening metathesis (romp) pathway.Turk J Chem. 2021 Aug 27;45(4):986-1003. doi: 10.3906/kim-2012-14. eCollection 2021. Turk J Chem. 2021. PMID: 34707429 Free PMC article.
-
Activity of Scorpion Venom-Derived Antifungal Peptides against Planktonic Cells of Candida spp. and Cryptococcus neoformans and Candida albicans Biofilms.Front Microbiol. 2016 Nov 18;7:1844. doi: 10.3389/fmicb.2016.01844. eCollection 2016. Front Microbiol. 2016. PMID: 27917162 Free PMC article.
-
A review on small molecular mimics of antimicrobial peptides with an emphasis on the structure-activity relationship perspective.RSC Med Chem. 2025 Jun 24. doi: 10.1039/d5md00407a. Online ahead of print. RSC Med Chem. 2025. PMID: 40656837 Free PMC article. Review.
-
Optimal Hydrophobicity in Ring-Opening Metathesis Polymerization-Based Protein Mimics Required for siRNA Internalization.Biomacromolecules. 2016 Jun 13;17(6):1969-77. doi: 10.1021/acs.biomac.6b00138. Epub 2016 May 19. Biomacromolecules. 2016. PMID: 27103189 Free PMC article.
-
Norbornane-based cationic antimicrobial peptidomimetics targeting the bacterial membrane.Eur J Med Chem. 2018 Dec 5;160:9-22. doi: 10.1016/j.ejmech.2018.09.072. Epub 2018 Oct 3. Eur J Med Chem. 2018. PMID: 30316060 Free PMC article.
References
-
- Neu HC. The crisis in antibiotic resistance. Science. 1992;257:1064–73. - PubMed
-
- Brown K, Hancock R. Cationic host defense (antimicrobial) peptides. Current Opinion in Immunology. 2006;18:24–30. - PubMed
-
- McPhee JB, Hancock REW. Function and therapeutic potential of host defence peptides. Journal of Peptide Science. 2005;11:677–687. - PubMed
-
- Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

