Enantioselective α-arylation of aldehydes via the productive merger of iodonium salts and organocatalysis
- PMID: 21388207
- PMCID: PMC3082494
- DOI: 10.1021/ja2008906
Enantioselective α-arylation of aldehydes via the productive merger of iodonium salts and organocatalysis
Abstract
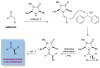
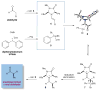
The enantioselective α-arylation of aldehydes has been accomplished using diaryliodonium salts and a combination of copper and organic catalysts. These mild catalytic conditions provide a new strategy for the enantioselective construction and retention of enolizable α-formyl benzylic stereocenters, a valuable synthon for the production of medicinal agents. As one example, this new asymmetric protocol has been applied to the rapid synthesis of (S)-ketoprofen, a commercially successful oral and topical analgesic.
Figures
References
-
-
For racemic α-arylation of carbonyl compounds, see: Muratake H, Nakai H. Tetrahedron Lett. 1999;40:2355.Martín R, Buchwald SL. Angew Chem, Int Ed. 2007;46:7236.Vo GC, Hartwig JF. Angew Chem, Int Ed. 2008;47:2127.
-
-
-
For asymmetric α-arylation of carbonyl compounds, see: García-Fortanet J, Buchwald SL. Angew Chem, Int Ed. 2008;47:8108.Taylor AM, Altman RA, Buchwald SL. J Am Chem Soc. 2009;131:9900.Liao X, Weng Z, Hartwig JF. J Am Chem Soc. 2008;130:195.
-
-
-
For reviews on diaryliodonium salts, see Merritt EA, Olofsson B. Angew Chem, Int Ed. 2009;48:9052.Varvoglis A. The Organic Chemistry of Polycoordinated Iodine. VCH Publishers; New York: 1992. Grushin VV. Chem Soc Rev. 2000;29:315.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials