Blood-based detection of radiation exposure in humans based on novel phospho-Smc1 ELISA
- PMID: 21388270
- PMCID: PMC3123689
- DOI: 10.1667/RR2402.1
Blood-based detection of radiation exposure in humans based on novel phospho-Smc1 ELISA
Abstract
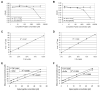
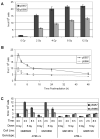
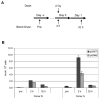
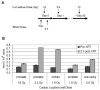
The structural maintenance of chromosome 1 (Smc1) protein is a member of the highly conserved cohesin complex and is involved in sister chromatid cohesion. In response to ionizing radiation, Smc1 is phosphorylated at two sites, Ser-957 and Ser-966, and these phosphorylation events are dependent on the ATM protein kinase. In this study, we describe the generation of two novel ELISAs for quantifying phospho-Smc1(Ser-957) and phospho-Smc1(Ser-966). Using these novel assays, we quantify the kinetic and biodosimetric responses of human cells of hematological origin, including immortalized cells, as well as both quiescent and cycling primary human PBMC. Additionally, we demonstrate a robust in vivo response for phospho-Smc1(Ser-957) and phospho-Smc1(Ser-966) in lymphocytes of human patients after therapeutic exposure to ionizing radiation, including total-body irradiation, partial-body irradiation, and internal exposure to (131)I. These assays are useful for quantifying the DNA damage response in experimental systems and potentially for the identification of individuals exposed to radiation after a radiological incident.
Figures




Similar articles
-
Involvement of the cohesin protein, Smc1, in Atm-dependent and independent responses to DNA damage.Genes Dev. 2002 Mar 1;16(5):560-70. doi: 10.1101/gad.970602. Genes Dev. 2002. PMID: 11877376 Free PMC article.
-
Radiation-Induced Phosphorylation of Serine 360 of SMC1 in Human Peripheral Blood Mononuclear Cells.Radiat Res. 2019 Mar;191(3):262-270. doi: 10.1667/RR15179.1. Epub 2019 Jan 31. Radiat Res. 2019. PMID: 30702968
-
SMC1 is a downstream effector in the ATM/NBS1 branch of the human S-phase checkpoint.Genes Dev. 2002 Mar 1;16(5):571-82. doi: 10.1101/gad.970702. Genes Dev. 2002. PMID: 11877377 Free PMC article.
-
The ATM-dependent DNA damage signaling pathway.Cold Spring Harb Symp Quant Biol. 2005;70:99-109. doi: 10.1101/sqb.2005.70.002. Cold Spring Harb Symp Quant Biol. 2005. PMID: 16869743 Review.
-
Ataxia-telangiectasia-like disorder (ATLD)-its clinical presentation and molecular basis.DNA Repair (Amst). 2004 Aug-Sep;3(8-9):1219-25. doi: 10.1016/j.dnarep.2004.04.009. DNA Repair (Amst). 2004. PMID: 15279810 Review.
Cited by
-
Modeling and experiments of magneto-nanosensors for diagnostics of radiation exposure and cancer.Biomed Microdevices. 2013 Aug;15(4):665-671. doi: 10.1007/s10544-012-9678-z. Biomed Microdevices. 2013. PMID: 22763391 Free PMC article.
-
Transcript Analysis for Internal Biodosimetry Using Peripheral Blood from Neuroblastoma Patients Treated with (131)I-mIBG, a Targeted Radionuclide.Radiat Res. 2016 Sep;186(3):235-44. doi: 10.1667/RR14263.1. Epub 2016 Aug 24. Radiat Res. 2016. PMID: 27556353 Free PMC article.
-
Structural Maintenance of Chromosomes protein 1: Role in Genome Stability and Tumorigenesis.Int J Biol Sci. 2017 Sep 3;13(8):1092-1099. doi: 10.7150/ijbs.21206. eCollection 2017. Int J Biol Sci. 2017. PMID: 28924389 Free PMC article. Review.
-
SMC1A is associated with radioresistance in prostate cancer and acts by regulating epithelial-mesenchymal transition and cancer stem-like properties.Mol Carcinog. 2019 Jan;58(1):113-125. doi: 10.1002/mc.22913. Epub 2018 Oct 5. Mol Carcinog. 2019. PMID: 30242889 Free PMC article.
-
An ever-changing landscape in Roberts syndrome biology: Implications for macromolecular damage.PLoS Genet. 2020 Dec 31;16(12):e1009219. doi: 10.1371/journal.pgen.1009219. eCollection 2020 Dec. PLoS Genet. 2020. PMID: 33382686 Free PMC article. Review.
References
-
- Waselenko JK, MacVittie TJ, Blakely WF, Pesik N, Wiley AL, Dickerson WE, Tsu H, Confer DL, Coleman CN, Dainiak N. Medical management of the acute radiation syndrome: Recommendations of the strategic national stockpile radiation working group. Ann. Intern. Med. 2004;140:1037–1051. - PubMed
-
- Grayson JM, Harrington LE, Lanier JG, Wherry EJ, Ahmed R. Differential sensitivity of naive and memory CD8+ Tcells to apoptosis in vivo. J. Immunol. 2002;169:3760–3770. - PubMed
-
- Cui YF, Gao YB, Yang H, Xiong CQ, Xia GW, Wang DW. Apoptosis of circulating lymphocytes induced by whole body gamma-irradiation and its mechanism. J. Environ. Pathol. Toxicol. Oncol. 1999;18:185–189. - PubMed
-
- Grace MB, Muderhwa JM, Salter CA, Blakely WF. Use of a centrifuge-based automated blood cell counter for radiation dose assessment. Mil. Med. 2006;171:908–912. - PubMed
-
- Goans RE, Holloway EC, Berger ME, Ricks RC. Early dose assessment following severe radiation accidents. Health Phys. 1997;72:513–518. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

