Pentoxifylline enhances the radioprotective properties of γ-tocotrienol: differential effects on the hematopoietic, gastrointestinal and vascular systems
- PMID: 21388273
- PMCID: PMC3115470
- DOI: 10.1667/RR2399.1
Pentoxifylline enhances the radioprotective properties of γ-tocotrienol: differential effects on the hematopoietic, gastrointestinal and vascular systems
Abstract
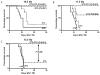
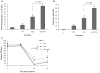
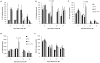
The vitamin E analog γ-tocotrienol (GT3) is a potent radioprotector and mitigator. This study was performed to (a) determine whether the efficacy of GT3 can be enhanced by the addition of the phosphodiesterase inhibitor pentoxifylline (PTX) and (b) to obtain information about the mechanism of action. Mice were injected subcutaneously with vehicle, GT3 [400 mg/kg 24 h before total-body irradiation (TBI)], PTX (200 mg/kg 30 min before TBI), or GT3+PTX before being exposed to 8.5-13 Gy TBI. Overall lethality, survival time and intestinal, hematopoietic and vascular injury were assessed. Cytokine levels in the bone marrow microenvironment were measured, and the requirement for endothelial nitric oxide synthase (eNOS) was studied in eNOS-deficient mice. GT3+PTX significantly improved survival compared to GT3 alone and provided full protection against lethality even after exposure to 12.5 Gy. GT3+PTX improved bone marrow CFUs, spleen colony counts and platelet recovery compared to GT3 alone. GT3 and GT3+PTX increased bone marrow plasma G-CSF levels as well as the availability of IL-1α, IL-6 and IL-9 in the early postirradiation phase. GT3 and GT3+PTX were equally effective in ameliorating intestinal injury and vascular peroxynitrite production. Survival studies in eNOS-deficient mice and appropriate controls revealed that eNOS was not required for protection against lethality after TBI. Combined treatment with GT3 and PTX increased postirradiation survival over that with GT3 alone by a mechanism that may depend on induction of hematopoietic stimuli. GT3+PTX did not reduce GI toxicity or vascular oxidative stress compared to GT3 alone. The radioprotective effect of either drug alone or both drugs in combination does not require the presence of eNOS.
Figures
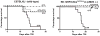

Similar articles
-
gamma-Tocotrienol ameliorates intestinal radiation injury and reduces vascular oxidative stress after total-body irradiation by an HMG-CoA reductase-dependent mechanism.Radiat Res. 2009 May;171(5):596-605. doi: 10.1667/RR1632.1. Radiat Res. 2009. PMID: 19580495 Free PMC article.
-
Reduction of radiation-induced vascular nitrosative stress by the vitamin E analog γ-tocotrienol: evidence of a role for tetrahydrobiopterin.Int J Radiat Oncol Biol Phys. 2011 Mar 1;79(3):884-91. doi: 10.1016/j.ijrobp.2010.08.032. Epub 2010 Oct 13. Int J Radiat Oncol Biol Phys. 2011. PMID: 20950957 Free PMC article.
-
γ-Tocotrienol-Loaded Liposomes for Radioprotection from Hematopoietic Side Effects Caused by Radiotherapeutic Drugs.J Nucl Med. 2021 Apr;62(4):584-590. doi: 10.2967/jnumed.120.244681. Epub 2020 Aug 21. J Nucl Med. 2021. PMID: 32826318 Free PMC article.
-
γ-Tocotrienol as a Promising Countermeasure for Acute Radiation Syndrome: Current Status.Int J Mol Sci. 2016 May 3;17(5):663. doi: 10.3390/ijms17050663. Int J Mol Sci. 2016. PMID: 27153057 Free PMC article. Review.
-
Hematological targets of radiation damage.Curr Drug Targets. 2010 Nov;11(11):1375-85. doi: 10.2174/1389450111009011375. Curr Drug Targets. 2010. PMID: 20583980 Review.
Cited by
-
Vitamin E: tocopherols and tocotrienols as potential radiation countermeasures.J Radiat Res. 2013 Nov 1;54(6):973-88. doi: 10.1093/jrr/rrt048. Epub 2013 May 8. J Radiat Res. 2013. PMID: 23658414 Free PMC article. Review.
-
Endothelial progenitor cells in the host defense response.Pharmacol Ther. 2023 Jan;241:108315. doi: 10.1016/j.pharmthera.2022.108315. Epub 2022 Nov 24. Pharmacol Ther. 2023. PMID: 36436689 Free PMC article. Review.
-
Effects of late administration of pentoxifylline and tocotrienols in an image-guided rat model of localized heart irradiation.PLoS One. 2013 Jul 22;8(7):e68762. doi: 10.1371/journal.pone.0068762. Print 2013. PLoS One. 2013. PMID: 23894340 Free PMC article.
-
Radioprotection and Radiomitigation: From the Bench to Clinical Practice.Biomedicines. 2020 Oct 30;8(11):461. doi: 10.3390/biomedicines8110461. Biomedicines. 2020. PMID: 33142986 Free PMC article. Review.
-
Nuclear and Radiological Emergencies: Biological Effects, Countermeasures and Biodosimetry.Antioxidants (Basel). 2022 May 31;11(6):1098. doi: 10.3390/antiox11061098. Antioxidants (Basel). 2022. PMID: 35739995 Free PMC article. Review.
References
-
- Ghosh SP, Kulkarni S, Hieber K, Toles R, Romanyukha L, Kao TC, Hauer-Jensen M, Kumar KS. Gamma-tocotrienol, a tocol antioxidant as a potent radioprotector. Int J Radiat Biol. 2009;85:598–606. - PubMed
-
- Kulkarni S, Ghosh SP, Satyamitra M, Mog S, Hieber K, Romanyukha L, Gambles K, Toles R, Kao TC, Kumar KS. Gamma-tocotrienol protects hematopoietic stem and progenitor cells in mice after total-body irradiation. Radiat Res. 2010;173:738–747. - PubMed
-
- Bese NS, Munzuroglu F, Uslu B, Arbak S, Yesiladali G, Sut N, Altug T, Ober A. Vitamin E protects against the development of radiation-induced pulmonary fibrosis in rats. Clin Oncol (R Coll Radiol) 2007;19:260–264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

