Endoplasmic reticulum stress in wake-active neurons progresses with aging
- PMID: 21388495
- PMCID: PMC3125474
- DOI: 10.1111/j.1474-9726.2011.00699.x
Endoplasmic reticulum stress in wake-active neurons progresses with aging
Abstract
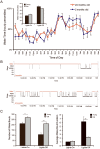
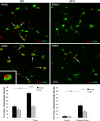
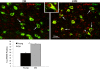
Fragmentation of wakefulness and sleep are expected outcomes of advanced aging. We hypothesize that wake neurons develop endoplasmic reticulum dyshomeostasis with aging, in parallel with impaired wakefulness. In this series of experiments, we sought to more fully characterize age-related changes in wakefulness and then, in relevant wake neuronal populations, explore functionality and endoplasmic reticulum homeostasis. We report that old mice show greater sleep/wake transitions in the active period with markedly shortened wake periods, shortened latencies to sleep, and less wake time in the subjective day in response to a novel social encounter. Consistent with sleep/wake instability and reduced social encounter wakefulness, orexinergic and noradrenergic wake neurons in aged mice show reduced c-fos response to wakefulness and endoplasmic reticulum dyshomeostasis with increased nuclear translocation of CHOP and GADD34. We have identified an age-related unfolded protein response injury to and dysfunction of wake neurons. It is anticipated that these changes contribute to sleep/wake fragmentation and cognitive impairment in aging.
© 2011 The Authors. Aging Cell © 2011 Blackwell Publishing Ltd/Anatomical Society of Great Britain and Ireland.
Figures
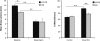
References
-
- Abbott RD, Ross GW, White LR, Tanner CM, Masaki KH, Nelson JS, Curb JD, Petrovitch H. Excessive daytime sleepiness and subsequent development of Parkinson disease. Neurology. 2005;65:1442–1446. - PubMed
-
- Bliwise DL, Ansari FP, Straight LB, Parker KP. Age changes in timing and 24-hour distribution of self-reported sleep. Am J Geriatr Psychiatry. 2005;13:1077–1082. - PubMed
-
- Brody H. The nervous system and aging. Adv Pathobiol. 1980;7:200–209. - PubMed
-
- Brush MH, Weiser DC, Shenolikar S. Growth arrest and DNA damage-inducible protein GADD34 targets protein phosphatase 1 alpha to the endoplasmic reticulum and promotes dephosphorylation of the alpha subunit of eukaryotic translation initiation factor 2. Molecular & Cellular Biology. 2003;23:1292–1303. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

