Comparison of the efficacy of tenofovir and adefovir in the treatment of chronic hepatitis B: a systematic review
- PMID: 21388525
- PMCID: PMC3063232
- DOI: 10.1186/1743-422X-8-111
Comparison of the efficacy of tenofovir and adefovir in the treatment of chronic hepatitis B: a systematic review
Abstract
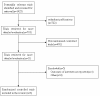
Chronic viral hepatitis B remains a global public health concern. Currently, several drugs, such as tenofovir and adefovir, are recommended for treatment of patients with chronic hepatitis B. tenofovir is a nucleoside analog with selective activity against hepatitis b virus and has been shown to be more potent in vitro than adefovir. But the results of trials comparing tenofovir and adefovir in the treatment of chronic hepatitis B were inconsistent. However, there was no systematic review on the comparison of the efficacy of tenofovir and adefovir in the treatment of chronic hepatitis B. To evaluate the comparison of the efficacy of tenofovir and adefovir in the treatment of chronic hepatitis B we conducted a systematic review and meta-analysis of clinical trials. We searched PUBMED, Web of Science, EMBASE, CNKI, VIP database, WANFANG database, the Cochrane Central Register of Controlled Trials and the Cochrane Database of Systematic Review. Finally six studies were left for analysis which involved 910 patients in total, of whom 576 were included in tenofovir groups and 334 were included in adefovir groups. At the end of 48-week treatment, tenofovir was superior to adefovir at the HBV-DNA suppression in patients[RR = 2.59; 95%CI(1.01-6.67), P = 0.05]. While there was no significant difference in the ALT normalization[RR = 1.15; 95%CI(0.96-1.37), P = 0.14], HBeAg seroconversion[RR = 1.32; 95%CI(1.00-1.75), P = 0.05] and HBsAg loss rate[RR = 1.19; 95%CI(0.74-1.91), P = 0.48]. More high-quality, well-designed, randomized controlled, multi-center trails are clearly needed to guide evolving standards of care for chronic hepatitis B.
Figures
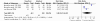
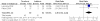
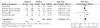
Similar articles
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.Health Technol Assess. 2006 Aug;10(28):iii-iv, xi-xiv, 1-183. doi: 10.3310/hta10280. Health Technol Assess. 2006. PMID: 16904047
-
Lamivudine plus adefovir combination therapy versus entecavir monotherapy for lamivudine-resistant chronic hepatitis B: a systematic review and meta-analysis.Virol J. 2011 Aug 8;8:393. doi: 10.1186/1743-422X-8-393. Virol J. 2011. PMID: 21824397 Free PMC article.
-
Comparison of the efficacy of Lamivudine plus adefovir versus entecavir in the treatment of Lamivudine-resistant chronic hepatitis B: a systematic review and meta-analysis.Clin Ther. 2013 Dec;35(12):1997-2006. doi: 10.1016/j.clinthera.2013.10.002. Epub 2013 Nov 13. Clin Ther. 2013. PMID: 24238791
-
Phyllanthus species for chronic hepatitis B virus infection.Cochrane Database Syst Rev. 2011 Apr 13;(4):CD008960. doi: 10.1002/14651858.CD008960.pub2. Cochrane Database Syst Rev. 2011. PMID: 21491412
-
Adefovir dipivoxil and pegylated interferon alpha for the treatment of chronic hepatitis B: an updated systematic review and economic evaluation.Health Technol Assess. 2009 Jul;13(35):1-172, iii. doi: 10.3310/hta13350. Health Technol Assess. 2009. PMID: 19607759
Cited by
-
Molecular characteristics of Hepatitis B and chronic liver disease in a cohort of HB carriers from Bamako, Mali.BMC Infect Dis. 2015 Apr 11;15:180. doi: 10.1186/s12879-015-0916-x. BMC Infect Dis. 2015. PMID: 25886382 Free PMC article.
-
Vitamin D deficiency aggravates nephrotoxicity, hypertension and dyslipidemia caused by tenofovir: role of oxidative stress and renin-angiotensin system.PLoS One. 2014 Jul 21;9(7):e103055. doi: 10.1371/journal.pone.0103055. eCollection 2014. PLoS One. 2014. PMID: 25048368 Free PMC article.
-
N6-Methyladenosine and Viral Infection.Front Microbiol. 2019 Mar 5;10:417. doi: 10.3389/fmicb.2019.00417. eCollection 2019. Front Microbiol. 2019. PMID: 30891023 Free PMC article. Review.
-
Imaging of Hepatitis B Virus Nucleic Acids: Current Advances and Challenges.Viruses. 2022 Mar 8;14(3):557. doi: 10.3390/v14030557. Viruses. 2022. PMID: 35336964 Free PMC article. Review.
References
-
- Safioleas M, Lygidakis NJ, Manti C. Hepatitis B today. Hepatogastroenterology. 2007;54:545–548. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

