Examination of thromboxane synthase as a prognostic factor and therapeutic target in non-small cell lung cancer
- PMID: 21388528
- PMCID: PMC3074522
- DOI: 10.1186/1476-4598-10-25
Examination of thromboxane synthase as a prognostic factor and therapeutic target in non-small cell lung cancer
Abstract
Background: Thromboxane synthase (TXS) metabolises prostaglandin H2 into thromboxanes, which are biologically active on cancer cells. TXS over-expression has been reported in a range of cancers, and associated with a poor prognosis. TXS inhibition induces cell death in-vitro, providing a rationale for therapeutic intervention. We aimed to determine the expression profile of TXS in NSCLC and if it is prognostic and/or a survival factor in the disease.
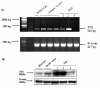
Methods: TXS expression was examined in human NSCLC and matched controls by western analysis and IHC. TXS metabolite (TXB2) levels were measured by EIA. A 204-patient NSCLC TMA was stained for COX-2 and downstream TXS expression. TXS tissue expression was correlated with clinical parameters, including overall survival. Cell proliferation/survival and invasion was examined in NSCLC cells following both selective TXS inhibition and stable TXS over-expression.
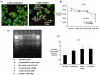
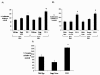
Results: TXS was over-expressed in human NSCLC samples, relative to matched normal controls. TXS and TXB2 levels were increased in protein (p < 0.05) and plasma (p < 0.01) NSCLC samples respectively. TXS tissue expression was higher in adenocarcinoma (p < 0.001) and female patients (p < 0.05). No significant correlation with patient survival was observed. Selective TXS inhibition significantly reduced tumour cell growth and increased apoptosis, while TXS over-expression stimulated cell proliferation and invasiveness, and was protective against apoptosis.
Conclusion: TXS is over-expressed in NSCLC, particularly in the adenocarcinoma subtype. Inhibition of this enzyme inhibits proliferation and induces apoptosis. Targeting thromboxane synthase alone, or in combination with conventional chemotherapy is a potential therapeutic strategy for NSCLC.
Figures


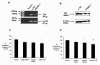

References
-
- Needleman P, Isakson PC. Selective inhibition of cyclooxygenase-2. Science and Medicine. 1998. pp. 26–35.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

