Environmentally persistent free radicals induce airway hyperresponsiveness in neonatal rat lungs
- PMID: 21388553
- PMCID: PMC3061909
- DOI: 10.1186/1743-8977-8-11
Environmentally persistent free radicals induce airway hyperresponsiveness in neonatal rat lungs
Abstract
Background: Increased asthma risk/exacerbation in children and infants is associated with exposure to elevated levels of ultrafine particulate matter (PM). The presence of a newly realized class of pollutants, environmentally persistent free radicals (EPFRs), in PM from combustion sources suggests a potentially unrecognized risk factor for the development and/or exacerbation of asthma.
Methods: Neonatal rats (7-days of age) were exposed to EPFR-containing combustion generated ultrafine particles (CGUFP), non-EPFR containing CGUFP, or air for 20 minutes per day for one week. Pulmonary function was assessed in exposed rats and age matched controls. Lavage fluid was isolated and assayed for cellularity and cytokines and in vivo indicators of oxidative stress. Pulmonary histopathology and characterization of differential protein expression in lung homogenates was also performed.
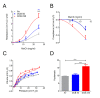
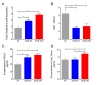
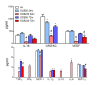
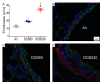
Results: Neonates exposed to EPFR-containing CGUFP developed significant pulmonary inflammation, and airway hyperreactivity. This correlated with increased levels of oxidative stress in the lungs. Using differential two-dimensional electrophoresis, we identified 16 differentially expressed proteins between control and CGUFP exposed groups. In the rats exposed to EPFR-containing CGUFP; peroxiredoxin-6, cofilin1, and annexin A8 were upregulated.
Conclusions: Exposure of neonates to EPFR-containing CGUFP induced pulmonary oxidative stress and lung dysfunction. This correlated with alterations in the expression of various proteins associated with the response to oxidative stress and the regulation of glucocorticoid receptor translocation in T lymphocytes.
Figures


References
-
- Boezen M, Schouten J, Rijcken B, Vonk J, Gerritsen J, van der Zee S, Hoek G, Brunekreef B, Postma D. Peak expiratory flow variability, bronchial responsiveness, and susceptibility to ambient air pollution in adults. Am J Respir Crit Care Med. 1998;158:1848–1854. - PubMed
-
- Schwartz J, Dockery DW, Neas LM, Wypij D, Ware JH, Spengler JD, Koutrakis P, Speizer FE, Ferris BG Jr. Acute effects of summer air pollution on respiratory symptom reporting in children. Am J Respir Crit Care Med. 1994;150:1234–1242. - PubMed
-
- Timonen KL, Pekkanen J. Air pollution and respiratory health among children with asthmatic or cough symptoms. Am J Respir Crit Care Med. 1997;156:546–552. - PubMed
-
- Vedal S, Petkau J, White R, Blair J. Acute effects of ambient inhalable particles in asthmatic and nonasthmatic children. Am J Respir Crit Care Med. 1998;157:1034–1043. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

