Antigen-specific IgA B memory cell responses to Shigella antigens elicited in volunteers immunized with live attenuated Shigella flexneri 2a oral vaccine candidates
- PMID: 21388888
- PMCID: PMC3078965
- DOI: 10.1016/j.clim.2011.02.003
Antigen-specific IgA B memory cell responses to Shigella antigens elicited in volunteers immunized with live attenuated Shigella flexneri 2a oral vaccine candidates
Abstract
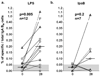
We studied the induction of antigen-specific IgA memory B cells (B(M)) in volunteers who received live attenuated Shigella flexneri 2a vaccines. Subjects ingested a single oral dose of 10(7), 10(8) or 10(9) CFU of S. flexneri 2a with deletions in guaBA (CVD 1204) or in guaBA, set and sen (CVD 1208). Antigen-specific serum and stool antibody responses to LPS and Ipa B were measured on days 0, 7, 14, 28 and 42. IgA B(M) cells specific to LPS, Ipa B and total IgA were assessed on days 0 and 28. We show the induction of significant LPS-specific IgA B(M) cells in anti-LPS IgA seroresponders. Positive correlations were found between anti-LPS IgA B(M) cells and anti-LPS IgA in serum and stool; IgA B(M) cell responses to IpaB were also observed. These B(M) cell responses are likely play an important role in modulating the magnitude and longevity of the humoral response.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Safety and immunogenicity of an intranasal Shigella flexneri 2a Invaplex 50 vaccine.Vaccine. 2011 Sep 16;29(40):7009-19. doi: 10.1016/j.vaccine.2011.07.033. Epub 2011 Jul 23. Vaccine. 2011. PMID: 21787825 Clinical Trial.
-
Antigen-specific B memory cell responses to lipopolysaccharide (LPS) and invasion plasmid antigen (Ipa) B elicited in volunteers vaccinated with live-attenuated Shigella flexneri 2a vaccine candidates.Vaccine. 2009 Jan 22;27(4):565-72. doi: 10.1016/j.vaccine.2008.10.081. Epub 2008 Nov 18. Vaccine. 2009. PMID: 19022324 Free PMC article.
-
Deletion in the Shigella enterotoxin genes further attenuates Shigella flexneri 2a bearing guanine auxotrophy in a phase 1 trial of CVD 1204 and CVD 1208.J Infect Dis. 2004 Nov 15;190(10):1745-54. doi: 10.1086/424680. Epub 2004 Oct 19. J Infect Dis. 2004. PMID: 15499528 Clinical Trial.
-
Antibody in Lymphocyte Supernatant (ALS) responses after oral vaccination with live Shigella sonnei vaccine candidates WRSs2 and WRSs3 and correlation with serum antibodies, ASCs, fecal IgA and shedding.PLoS One. 2021 Nov 18;16(11):e0259361. doi: 10.1371/journal.pone.0259361. eCollection 2021. PLoS One. 2021. PMID: 34793505 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of the live oral auxotrophic Shigella flexneri SFL124 in volunteers.Vaccine. 1992;10(6):395-404. doi: 10.1016/0264-410x(92)90070-z. Vaccine. 1992. PMID: 1598788 Clinical Trial.
Cited by
-
Diverse phosphorylation patterns of B cell receptor-associated signaling in naïve and memory human B cells revealed by phosphoflow, a powerful technique to study signaling at the single cell level.Front Cell Infect Microbiol. 2012 Oct 17;2:128. doi: 10.3389/fcimb.2012.00128. eCollection 2012. Front Cell Infect Microbiol. 2012. PMID: 23087912 Free PMC article.
-
Self-adjuvanting bacterial vectors expressing pre-erythrocytic antigens induce sterile protection against malaria.Front Immunol. 2013 Jul 4;4:176. doi: 10.3389/fimmu.2013.00176. eCollection 2013. Front Immunol. 2013. PMID: 23847617 Free PMC article.
-
Applying mathematical tools to accelerate vaccine development: modeling Shigella immune dynamics.PLoS One. 2013 Apr 2;8(4):e59465. doi: 10.1371/journal.pone.0059465. Print 2013. PLoS One. 2013. PMID: 23589755 Free PMC article.
-
Testing S. sonnei GMMA with and without Aluminium Salt-Based Adjuvants in Animal Models.Pharmaceutics. 2024 Apr 22;16(4):568. doi: 10.3390/pharmaceutics16040568. Pharmaceutics. 2024. PMID: 38675229 Free PMC article.
-
Further characterization of Shigella-specific (memory) B cells induced in healthy volunteer recipients of SF2a-TT15, a Shigella flexneri 2a synthetic glycan-based vaccine candidate.Front Immunol. 2023 Oct 31;14:1291664. doi: 10.3389/fimmu.2023.1291664. eCollection 2023. Front Immunol. 2023. PMID: 38022674 Free PMC article.
References
-
- Pickering LK. Antimicrobial resistance among enteric pathogens. Semin. Pediatr. Infect. Dis. 2004;15:71–77. - PubMed
-
- Bioterrorism Agents/Diseases. Center for Disease Control and Prevention Website. 2010. Available from: URL: http://www.bt.cdc.gov/agent/agentlist.asp.
-
- Bennish ML, Wojtyniak BJ. Mortality due to shigellosis: community and hospital data. Rev. Infect. Dis. 1991;13 Suppl 4:S245–S251. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

