Mes1 controls the meiosis I to meiosis II transition by distinctly regulating the anaphase-promoting complex/cyclosome coactivators Fzr1/Mfr1 and Slp1 in fission yeast
- PMID: 21389117
- PMCID: PMC3084671
- DOI: 10.1091/mbc.E10-09-0774
Mes1 controls the meiosis I to meiosis II transition by distinctly regulating the anaphase-promoting complex/cyclosome coactivators Fzr1/Mfr1 and Slp1 in fission yeast
Abstract
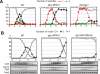
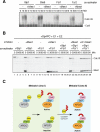
Meiosis is a specialized form of cell division generating haploid gametes and is dependent upon protein ubiquitylation by the anaphase-promoting complex/cyclosome (APC/C). Accurate control of the APC/C during meiosis is important in all eukaryotic cells and is in part regulated by the association of coactivators and inhibitors. We previously showed that the fission yeast meiosis-specific protein Mes1 binds to a coactivator and inhibits APC/C; however, regulation of the Mes1-mediated APC/C inhibition remains elusive. Here we show how Mes1 distinctively regulates different forms of the APC/C. We study all the coactivators present in the yeast genome and find that only Slp1/Cdc20 is essential for meiosis I progression. However, Fzr1/Mfr1 is a critical target for Mes1 inhibition because fzr1Δ completely rescues the defect on the meiosis II entry in mes1Δ cells. Furthermore, cell-free studies suggest that Mes1 behaves as a pseudosubstrate for Fzr1/Mfr1 but works as a competitive substrate for Slp1. Intriguingly, mutations in the D-box or KEN-box of Mes1 increase its recognition as a substrate by Fzr1, but not by Slp1. Thus Mes1 interacts with two coactivators in a different way to control the activity of the APC/C required for the meiosis I/meiosis II transition.
Figures






References
-
- Asakawa H, Kitamura K, Shimoda C. A novel Cdc20-related WD-repeat protein, Fzr1, is required for spore formation in Schizosaccharomyces pombe. Mol Genet Genomics. 2001;265:424–435. - PubMed
-
- Bähler J, Wu JQ, Longtine MS, Shah NG, McKenzie A, III, Steever AB, Wach A, Philippsen P, Pringle JR. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast. 1998;14:943–951. - PubMed
-
- Blanco MA, Pelloquin L, Moreno S. Fission yeast mfr1 activates APC and coordinates meiotic nuclear division with sporulation. J Cell Sci. 2001;114:2135–2143. - PubMed
-
- Chu T, Henrion G, Haegeli V, Strickland S. Cortex, a Drosophila gene required to complete oocyte meiosis, is a member of the Cdc20/Fizzy protein family. Genesis. 2001;29:141–152. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

