Open reading frames carried on UL/b' are implicated in shedding and horizontal transmission of rhesus cytomegalovirus in rhesus monkeys
- PMID: 21389128
- PMCID: PMC3126184
- DOI: 10.1128/JVI.02631-10
Open reading frames carried on UL/b' are implicated in shedding and horizontal transmission of rhesus cytomegalovirus in rhesus monkeys
Abstract
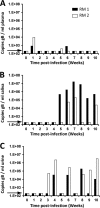
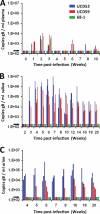
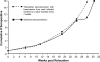
Implicit with the use of animal models to test human cytomegalovirus (HCMV) vaccines is the assumption that the viral challenge of vaccinated animals reflects the anticipated virus-host interactions following exposure of vaccinated humans to HCMV. Variables of animal vaccine studies include the route of exposure to and the titer of challenge virus, as well as the genomic coding content of the challenge virus. This study was initiated to provide a better context for conducting vaccine trials with nonhuman primates by determining whether the in vivo phenotype of culture-passaged strains of rhesus cytomegalovirus (RhCMV) is comparable to that of wild-type RhCMV (RhCMV-WT), particularly in relation to the shedding of virus into bodily fluids and the potential for horizontal transmission. Results of this study demonstrate that two strains containing a full-length UL/b' region of the RhCMV genome, which encodes proteins involved in epithelial tropism and immune evasion, were persistently shed in large amounts in bodily fluids and horizontally transmitted, whereas a strain lacking a complete UL/b' region was not shed or transmitted to cagemates. Shedding patterns exhibited by strains encoding a complete UL/b' region were consistent with patterns observed in naturally infected monkeys, the majority of whom persistently shed high levels of virus in saliva for extended periods of time after seroconversion. Frequent viral shedding contributed to a high rate of infection, with RhCMV-infected monkeys transmitting virus to one naïve animal every 7 weeks after introduction of RhCMV-WT into an uninfected cohort. These results demonstrate that the RhCMV model can be designed to rigorously reflect the challenges facing HCMV vaccine trials, particularly those related to horizontal transmission.
Figures
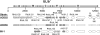

Similar articles
-
Limited dissemination and shedding of the UL128 complex-intact, UL/b'-defective rhesus cytomegalovirus strain 180.92.J Virol. 2014 Aug;88(16):9310-20. doi: 10.1128/JVI.00162-14. Epub 2014 Jun 4. J Virol. 2014. PMID: 24899204 Free PMC article.
-
Pathogenesis of Wild-Type-Like Rhesus Cytomegalovirus Strains following Oral Exposure of Immune-Competent Rhesus Macaques.J Virol. 2022 Feb 9;96(3):e0165321. doi: 10.1128/JVI.01653-21. Epub 2021 Nov 17. J Virol. 2022. PMID: 34788083 Free PMC article.
-
Vaccine-induced control of viral shedding following rhesus cytomegalovirus challenge in rhesus macaques.J Virol. 2011 Mar;85(6):2878-90. doi: 10.1128/JVI.00883-10. Epub 2010 Dec 29. J Virol. 2011. PMID: 21191005 Free PMC article.
-
The power of human cytomegalovirus (HCMV) hijacked UL/b' functions lost in vitro.Acta Virol. 2020;64(2):117-130. doi: 10.4149/av_2020_202. Acta Virol. 2020. PMID: 32551781 Review.
-
Rhesus cytomegalovirus a nonhuman primate model for the study of human cytomegalovirus.Adv Virus Res. 2008;72:207-26. doi: 10.1016/S0065-3527(08)00405-3. Adv Virus Res. 2008. PMID: 19081492 Review.
Cited by
-
A vaccine based on the rhesus cytomegalovirus UL128 complex induces broadly neutralizing antibodies in rhesus macaques.J Virol. 2013 Feb;87(3):1322-32. doi: 10.1128/JVI.01669-12. Epub 2012 Nov 14. J Virol. 2013. PMID: 23152525 Free PMC article.
-
Coding potential of UL/b' from the initial source of rhesus cytomegalovirus Strain 68-1.Virology. 2013 Dec;447(1-2):208-12. doi: 10.1016/j.virol.2013.08.026. Epub 2013 Oct 2. Virology. 2013. PMID: 24210116 Free PMC article.
-
Nonhuman primate models of pediatric viral diseases.Front Cell Infect Microbiol. 2024 Dec 3;14:1493885. doi: 10.3389/fcimb.2024.1493885. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39691699 Free PMC article. Review.
-
Exploitation of Interleukin-10 (IL-10) Signaling Pathways: Alternate Roles of Viral and Cellular IL-10 in Rhesus Cytomegalovirus Infection.J Virol. 2016 Oct 14;90(21):9920-9930. doi: 10.1128/JVI.00635-16. Print 2016 Nov 1. J Virol. 2016. PMID: 27558431 Free PMC article.
-
Neutralization of rhesus cytomegalovirus IL-10 reduces horizontal transmission and alters long-term immunity.Proc Natl Acad Sci U S A. 2019 Jun 25;116(26):13036-13041. doi: 10.1073/pnas.1903317116. Epub 2019 Jun 12. Proc Natl Acad Sci U S A. 2019. PMID: 31189602 Free PMC article.
References
-
- Adler B., et al. 2006. Role of human cytomegalovirus UL131A in cell type-specific virus entry and release. J. Gen. Virol. 87:2451–2460 - PubMed
-
- Adler S. P. 1991. Cytomegalovirus and child day care: risk factors for maternal infection. Pediatr. Infect. Dis. J. 10:590–594 - PubMed
-
- Adler S. P. 1991. Molecular epidemiology of cytomegalovirus: a study of factors affecting transmission among children at three day-care centers. Pediatr. Infect. Dis. J. 10:584–590 - PubMed
-
- Adler S. P. 1986. Molecular epidemiology of cytomegalovirus: evidence for viral transmission to parents from children infected at a day care center. Pediatr. Infect. Dis. 5:315–318 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

