Functional relationships between agonist binding sites and coupling regions of homomeric Cys-loop receptors
- PMID: 21389221
- PMCID: PMC3907114
- DOI: 10.1523/JNEUROSCI.5940-10.2011
Functional relationships between agonist binding sites and coupling regions of homomeric Cys-loop receptors
Abstract
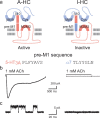
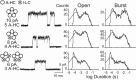
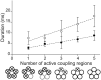
Each subunit in a homopentameric Cys-loop receptor contains a specialized coupling region positioned between the agonist binding domain and the ion conductive channel. To determine the contribution of each coupling region to the stability of the open channel, we constructed a receptor subunit (α7-5-HT(3A)) with both a disabled coupling region and a reporter mutation that alters unitary conductance, and coexpressed normal and mutant subunits. The resulting receptors show single-channel current amplitudes that are quantized according to the number of reporter mutations per receptor, allowing correlation of the number of intact coupling regions with mean open time. We find that each coupling region contributes an equal increment to the stability of the open channel. However, by altering the numbers and locations of active coupling regions and binding sites, we find that a coupling region in a subunit flanked by inactive binding sites can still stabilize the open channel. We also determine minimal requirements for channel opening regardless of stability and find that channel opening can occur in a receptor with one active coupling region flanked by functional binding sites or with one active binding site flanked by functional coupling regions. The overall findings show that, whereas the agonist binding sites contribute interdependently and asymmetrically to open-channel stability, the coupling regions contribute independently and symmetrically.
Figures





References
-
- Bartos M, Corradi J, Bouzat C. Structural basis of activation of Cys-loop receptors: the extracellular-transmembrane interface as a coupling region. Mol Neurobiol. 2009;40:236–252. - PubMed
-
- Beckstein O, Sansom MS. The influence of geometry, surface character, and flexibility on the permeation of ions and water through biological pores. Phys Biol. 2004;1:42–52. - PubMed
-
- Beckstein O, Sansom MS. A hydrophobic gate in an ion channel: the closed state of the nicotinic acetylcholine receptor. Phys Biol. 2006;3:147–159. - PubMed
-
- Bouzat C, Bren N, Sine SM. Structural basis of the different gating kinetics of fetal and adult acetylcholine receptors. Neuron. 1994;13:1395–1402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
