Experimental protocols alter phototransduction: the implications for retinal processing at visual threshold
- PMID: 21389222
- PMCID: PMC3063123
- DOI: 10.1523/JNEUROSCI.4750-10.2011
Experimental protocols alter phototransduction: the implications for retinal processing at visual threshold
Abstract
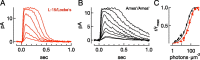
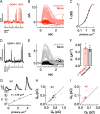
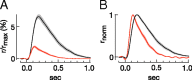
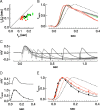
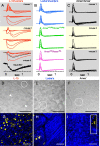
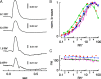
Vision in dim light, when photons are scarce, requires reliable signaling of the arrival of single photons. Rod photoreceptors accomplish this task through the use of a G-protein-coupled transduction cascade that amplifies the activity of single active rhodopsin molecules. This process is one of the best understood signaling cascades in biology, yet quantitative measurements of the amplitude and kinetics of the rod's response in mice vary by a factor of ∼ 2 across studies. What accounts for these discrepancies? We used several experimental approaches to reconcile differences in published properties of rod responses. First, we used suction electrode recordings from single rods to compare measurements across a range of recording conditions. Second, we compared measurements of single-cell photocurrents to estimates of rod function from in vitro electroretinograms. Third, we assayed the health of the post-receptor retinal tissue in these different conditions. Several salient points emerge from these experiments: (1) recorded responses can be altered dramatically by how the retina is stored; (2) the kinetics of the recovery of responses to bright but not dim flashes are strongly sensitive to the extracellular concentration of magnesium; (3) experimental conditions that produce very different single-photon responses measured in single rods produce near identical derived rod responses from the electroretinogram. The dependence of rod responses on experimental conditions will be a key consideration in efforts to extract general principles of G-protein signaling from studies of phototransduction and to relate these signals to downstream mechanisms that facilitate visual sensitivity.
Figures



References
-
- Aho AC, Donner K, Hydén C, Larsen LO, Reuter T. Low retinal noise in animals with low body temperature allows high visual sensitivity. Nature. 1988;334:348–350. - PubMed
-
- Ames A, 3rd, Nesbett FB. In vitro retina as an experimental model of the central nervous system. J Neurochem. 1981;37:867–877. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
