Cardiopulmonary function in two human disorders of the hypoxia-inducible factor (HIF) pathway: von Hippel-Lindau disease and HIF-2alpha gain-of-function mutation
- PMID: 21389259
- PMCID: PMC3159892
- DOI: 10.1096/fj.10-177378
Cardiopulmonary function in two human disorders of the hypoxia-inducible factor (HIF) pathway: von Hippel-Lindau disease and HIF-2alpha gain-of-function mutation
Abstract
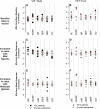
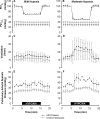
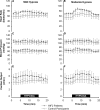
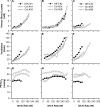
The hypoxia-inducible factors (HIFs; isoforms HIF-1α, HIF-2α, HIF-3α) mediate many responses to hypoxia. Their regulation is principally by oxygen-dependent degradation, which is initiated by hydroxylation of specific proline residues followed by binding of von Hippel-Lindau (VHL) protein. Chuvash polycythemia is a disorder with elevated HIF. It arises through germline homozygosity for hypomorphic VHL alleles and has a phenotype of hematological, cardiopulmonary, and metabolic abnormalities. This study explores the phenotype of two other HIF pathway diseases: classic VHL disease and HIF-2α gain-of-function mutation. No cardiopulmonary abnormalities were detected in classic VHL disease. HIF-2α gain-of-function mutations were associated with pulmonary hypertension, increased cardiac output, increased heart rate, and increased pulmonary ventilation relative to metabolism. Comparison of the HIF-2α gain-of-function responses with data from studies of Chuvash polycythemia suggested that other aspects of the Chuvash phenotype were diminished or absent. In classic VHL disease, patients are germline heterozygous for mutations in VHL, and the present results suggest that a single wild-type allele for VHL is sufficient to maintain normal cardiopulmonary function. The HIF-2α gain-of-function phenotype may be more limited than the Chuvash phenotype either because HIF-1α is not elevated in the former condition, or because other HIF-independent functions of VHL are perturbed in Chuvash polycythemia.
Figures
References
-
- Schofield C. J., Ratcliffe P. J. (2004) Oxygen sensing by HIF hydroxylases. Nat. Rev. Mol. Cell. Biol. 5, 343–354 - PubMed
-
- Semenza G. L. (2004) Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiology 19, 176–182 - PubMed
-
- Semenza G. L. (2007) Oxygen-dependent regulation of mitochondrial respiration by hypoxia-inducible factor 1. Biochem. J. 405, 1–9 - PubMed
-
- Wenger R. H. (2002) Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J. 16, 1151–1162 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

