Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus
- PMID: 21389263
- PMCID: PMC3399524
- DOI: 10.1126/scitranslmed.3001180
Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus
Abstract
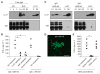
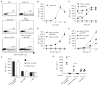
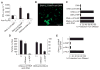
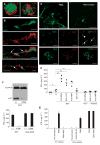
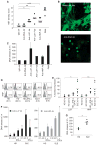
Systemic lupus erythematosus (SLE) is a severe and incurable autoimmune disease characterized by chronic activation of plasmacytoid dendritic cells (pDCs) and production of autoantibodies against nuclear self-antigens by hyperreactive B cells. Neutrophils are also implicated in disease pathogenesis; however, the mechanisms involved are unknown. Here, we identified in the sera of SLE patients immunogenic complexes composed of neutrophil-derived antimicrobial peptides and self-DNA. These complexes were produced by activated neutrophils in the form of web-like structures known as neutrophil extracellular traps (NETs) and efficiently triggered innate pDC activation via Toll-like receptor 9 (TLR9). SLE patients were found to develop autoantibodies to both the self-DNA and antimicrobial peptides in NETs, indicating that these complexes could also serve as autoantigens to trigger B cell activation. Circulating neutrophils from SLE patients released more NETs than those from healthy donors; this was further stimulated by the antimicrobial autoantibodies, suggesting a mechanism for the chronic release of immunogenic complexes in SLE. Our data establish a link between neutrophils, pDC activation, and autoimmunity in SLE, providing new potential targets for the treatment of this devastating disease.
Conflict of interest statement
Figures
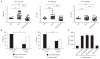
Comment in
-
Dissecting the immune cell mayhem that drives lupus pathogenesis.Sci Transl Med. 2011 Mar 9;3(73):73ps9. doi: 10.1126/scitranslmed.3002138. Sci Transl Med. 2011. PMID: 21389262 Free PMC article.
-
Autoimmunity: the role of neutrophils in SLE: untangling the NET.Nat Rev Rheumatol. 2011 May;7(5):252. doi: 10.1038/nrrheum.2011.41. Epub 2011 Apr 5. Nat Rev Rheumatol. 2011. PMID: 21468147 No abstract available.
-
TRAPping the cellular mechanisms of lupus.EMBO Mol Med. 2011 Oct;3(10):578-80. doi: 10.1002/emmm.201100167. Epub 2011 Sep 9. EMBO Mol Med. 2011. PMID: 21905225 Free PMC article. No abstract available.
References
-
- Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (α/β) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–336. - PubMed
-
- Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-α in systemic lupus erythematosus. Science. 2001;294:1540–1543. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

