Large shifts in pKa values of lysine residues buried inside a protein
- PMID: 21389271
- PMCID: PMC3069169
- DOI: 10.1073/pnas.1010750108
Large shifts in pKa values of lysine residues buried inside a protein
Abstract
Internal ionizable groups in proteins are relatively rare but they are essential for catalysis and energy transduction. To examine molecular determinants of their unusual and functionally important properties, we engineered 25 variants of staphylococcal nuclease with lysine residues at internal positions. Nineteen of the Lys residues have depressed pK(a) values, some as low as 5.3, and 20 titrate without triggering any detectable conformational reorganization. Apparently, simply by being buried in the protein interior, these Lys residues acquired pK(a) values comparable to those of naturally occurring internal ionizable groups involved in catalysis and biological H(+) transport. The pK(a) values of some of the internal Lys residues were affected by interactions with surface carboxylic groups. The apparent polarizability reported by the pK(a) values varied significantly from location to location inside the protein. These data will enable an unprecedented examination of the positional dependence of the dielectric response of a protein. This study also shows that the ability of proteins to withstand the presence of charges in their hydrophobic interior is a fundamental property inherent to all stable proteins, not a specialized adaptation unique to proteins that evolved to depend on internal charges for function.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
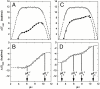
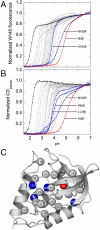
 ) of reference protein (Δ+PHS nuclease) (○) and its L125K variant (●) measured by GdnHCl denaturation monitored by Trp fluorescence. The line is from a simulation and it is only meant to guide the eye. (B) Difference in thermodynamic stability of Δ+PHS and the L125K variant (variant–reference). The line describes the fit of Eq. 2 to the data. The pKa of Lys-125 in folded (
) of reference protein (Δ+PHS nuclease) (○) and its L125K variant (●) measured by GdnHCl denaturation monitored by Trp fluorescence. The line is from a simulation and it is only meant to guide the eye. (B) Difference in thermodynamic stability of Δ+PHS and the L125K variant (variant–reference). The line describes the fit of Eq. 2 to the data. The pKa of Lys-125 in folded ( ) and denatured (
) and denatured ( ) states are indicated. (C) Thermodynamic stability of Δ+PHS nuclease (○) and of its T62K variant (●) measured by GdnHCl denaturation monitored by Trp fluorescence. The line is from a simulation and it is only meant to guide the eye. (D) Difference in thermodynamic stability of Δ+PHS and the T62K variant (variant–reference). The line describes the fit of Eq. 3 to the data. The pKa values relevant to Lys-62 (
) states are indicated. (C) Thermodynamic stability of Δ+PHS nuclease (○) and of its T62K variant (●) measured by GdnHCl denaturation monitored by Trp fluorescence. The line is from a simulation and it is only meant to guide the eye. (D) Difference in thermodynamic stability of Δ+PHS and the T62K variant (variant–reference). The line describes the fit of Eq. 3 to the data. The pKa values relevant to Lys-62 ( and
and  ) and the phenomenological pKa values (
) and the phenomenological pKa values ( and
and  ) of other ionizable side chain(s) affected by Lys-62 are indicated.
) of other ionizable side chain(s) affected by Lys-62 are indicated.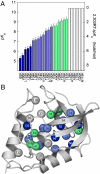

References
-
- Rastogi VK, Girvin ME. Structural changes linked to proton translocation by subunit c of the ATPase synthase. Nature. 1999;402:263–268. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

