p300 Acetyltransferase activity differentially regulates the localization and activity of the FOXO homologues in skeletal muscle
- PMID: 21389279
- PMCID: PMC3118617
- DOI: 10.1152/ajpcell.00255.2010
p300 Acetyltransferase activity differentially regulates the localization and activity of the FOXO homologues in skeletal muscle
Abstract
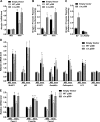
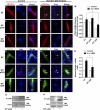
The Forkhead Box O (FOXO) transcription factors regulate diverse cellular processes, and in skeletal muscle are both necessary and sufficient for muscle atrophy. Although the regulation of FOXO by Akt is well evidenced in skeletal muscle, the current study demonstrates that FOXO is also regulated in muscle via the histone acetyltransferase (HAT) activities of p300/CREB-binding protein (CBP). Transfection of rat soleus muscle with a dominant-negative p300, which lacks HAT activity and inhibits endogenous p300 HAT activity, increased FOXO reporter activity and induced transcription from the promoter of a bona fide FOXO target gene, atrogin-1. Conversely, increased HAT activity via transfection of either wild-type (WT) p300 or WT CBP repressed FOXO activation in vivo in response to muscle disuse, and in C2C12 cells in response to dexamethasone and acute starvation. Importantly, manipulation of HAT activity differentially regulated the expression of various FOXO target genes. Cotransfection of FOXO1, FOXO3a, or FOXO4 with the p300 constructs further identified p300 HAT activity to also differentially regulate the activity of the FOXO homologues. Markedly, decreased HAT activity strongly increased FOXO3a transcriptional activity, while increased HAT activity repressed FOXO3a activity and prevented its nuclear localization in response to nutrient deprivation. In contrast, p300 increased FOXO1 nuclear localization. In summary, this study provides the first evidence to support the acetyltransferase activities of p300/CBP in regulating FOXO signaling in skeletal muscle and suggests that acetylation may be an important mechanism to differentially regulate the FOXO homologues and dictate which FOXO target genes are activated in response to varying atrophic stimuli.
Figures
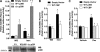



References
-
- Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704–1708, 2001 - PubMed
-
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96: 857–868, 1999 - PubMed
-
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303: 2011–2015, 2004 - PubMed
-
- Caiozzo VJ, Wu YZ, Baker MJ, Crumley R. Effects of denervation on cell cycle control in laryngeal muscle. Arch Otolaryngol Head Neck Surg 130: 1056–1068, 2004 - PubMed
-
- Chabi B, Adhihetty PJ, O'Leary MF, Menzies KJ, Hood DA. Relationship between Sirt1 expression and mitochondrial proteins during conditions of chronic muscle use and disuse. J Appl Physiol 107: 1730–1735, 2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

