Vasoactive intestinal polypeptide requires parallel changes in adenylate cyclase and phospholipase C to entrain circadian rhythms to a predictable phase
- PMID: 21389307
- PMCID: PMC3094187
- DOI: 10.1152/jn.00966.2010
Vasoactive intestinal polypeptide requires parallel changes in adenylate cyclase and phospholipase C to entrain circadian rhythms to a predictable phase
Abstract
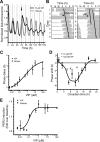
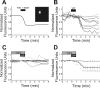
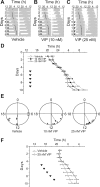
Circadian oscillations in the suprachiasmatic nucleus (SCN) depend on transcriptional repression by Period (PER)1 and PER2 proteins within single cells and on vasoactive intestinal polypeptide (VIP) signaling between cells. Because VIP is released by SCN neurons in a circadian pattern, and, after photic stimulation, it has been suggested to play a role in the synchronization to environmental light cycles. It is not known, however, if or how VIP entrains circadian gene expression or behavior. Here, we tested candidate signaling pathways required for VIP-mediated entrainment of SCN rhythms. We found that single applications of VIP reset PER2 rhythms in a time- and dose-dependent manner that differed from light. Unlike VIP-mediated signaling in other cell types, simultaneous antagonism of adenylate cyclase and phospholipase C activities was required to block the VIP-induced phase shifts of SCN rhythms. Consistent with this, VIP rapidly increased intracellular cAMP in most SCN neurons. Critically, daily VIP treatment entrained PER2 rhythms to a predicted phase angle within several days, depending on the concentration of VIP and the interval between VIP applications. We conclude that VIP entrains circadian timing among SCN neurons through rapid and parallel changes in adenylate cyclase and phospholipase C activities.
Figures

References
-
- Albers HE, Gillespie CF, Babagbemi TO, Huhman KL. Analysis of the phase shifting effects of gastrin releasing peptide when microinjected into the suprachiasmatic region. Neurosci Lett 191: 63–66, 1995. - PubMed
-
- Asai M, Yamaguchi S, Isejima H, Jonouchi M, Moriya T, Shibata S, Kobayashi M, Okamura H. Visualization of mPer1 transcription in vitro: NMDA induces a rapid phase shift of mPer1 gene in cultured SCN. Curr Biol 11: 1524–1527, 2001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

