IMiD immunomodulatory compounds block C/EBP{beta} translation through eIF4E down-regulation resulting in inhibition of MM
- PMID: 21389327
- PMCID: PMC3357942
- DOI: 10.1182/blood-2010-10-314278
IMiD immunomodulatory compounds block C/EBP{beta} translation through eIF4E down-regulation resulting in inhibition of MM
Abstract
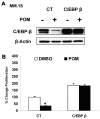
Immunomodulatory derivatives of thalidomide (IMiD compounds), such as pomalidomide and lenalidomide, are highly active in multiple myeloma (MM) treatment. However, the precise mechanisms of action and resistance in MM are unresolved. Here we show that IMiD compounds down-regulate CCAAT/enhancer-binding protein-β (C/EBPβ) resulting in abrogation of cell proliferation. Overexpression of C/EBPβ rescued MM cells from IMiD-induced inhibition of proliferation, indicating that C/EBPβ is critical in mediating antiproliferative effects. IMiD-induced decrease of C/EBPβ protein led to impaired transcription of interferon regulatory factor 4 (IRF4). Down-regulation of IRF4 by lenalidomide was confirmed by longitudinal studies of bone marrow samples from 23 patients obtained before and during lenalidomide treatment using CD138⁺/IRF4⁺ double labeling. In contrast to down-regulation of C/EBPβ protein, IMiD compounds did not alter C/EBPβ mRNA levels or protein stability, suggesting translational regulation of C/EBPβ. We could demonstrate that C/EBPβ protein expression is under eIF4E-translational control in MM. Furthermore, inhibition of the eIF4E-C/EBPβ axis by IMiD compounds was not observed in IMiD-resistant MM cells. However, targeting translation at a different level by inhibiting eukaryotic translation initiation factor 4E-binding protein 1 phosphorylation overcame resistance, suggesting that this pathway is critical and might be a target to overcome drug resistance.
Figures





References
-
- Lentzsch S, LeBlanc R, Podar K, et al. Immunomodulatory analogs of thalidomide inhibit growth of Hs Sultan cells and angiogenesis in vivo. Leukemia. 2003;17(1):41–44. - PubMed
-
- Lentzsch S, Rogers MS, LeBlanc R, et al. S-3-Amino-phthalimido-glutarimide inhibits angiogenesis and growth of B-cell neoplasias in mice. Cancer Res. 2002;62(8):2300–2305. - PubMed
-
- Escoubet-Lozach L, Lin IL, Jensen-Pergakes K, et al. Pomalidomide and lenalidomide induce p21 WAF-1 expression in both lymphoma and multiple myeloma through a LSD1-mediated epigenetic mechanism. Cancer Res. 2009;69(18):7347–7356. - PubMed
-
- Gandhi AK, Kang J, Capone L, et al. Dexamethasone synergizes with lenalidomide to inhibit multiple myeloma tumor growth, but reduces lenalidomide-induced immunomodulation of T and NK cell function. Curr Cancer Drug Targets. 2010;10(2):155–167. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

