Topical imiquimod has therapeutic and immunomodulatory effects against intracranial tumors
- PMID: 21389872
- PMCID: PMC3073686
- DOI: 10.1097/CJI.0b013e318209eed4
Topical imiquimod has therapeutic and immunomodulatory effects against intracranial tumors
Abstract
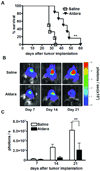
Topical imiquimod cream (trade name: Aldara) is a Toll-like receptor (TLR) 7 agonist that is approved for the treatment of cutaneous tumors. Aldara is also used as a vaccine adjuvant in clinical trials in patients with glioma and other tumors. The main mechanism of action ascribed to Aldara has been the local activation of TLR7(+) cells near the application site. Here we report the unexpected finding that Aldara has therapeutic and immunomodulatory activity as a single agent in mice bearing intracranial tumors. Repeated administration of Aldara onto the skin significantly increased the survival of mice bearing intracranial GL261 glioma and EMT6 breast carcinoma. Aldara treatment was associated with a reduction in the number CD4(+)Foxp3(+) regulatory T cells in the blood and brain tumor site. Mice treated with Aldara exhibited a generalized lymphopenia in the blood amidst an increase in brain tumor infiltrating CD4(+) and CD8(+) T cells and dendritic cells. Brain-infiltrating CD8(+) T cells were tumor reactive as demonstrated by degranulation and interferon-γ secretion in a GL261-dependent manner. In addition, soluble imiquimod directly inhibited the proliferation of GL261 cells in a TLR7-independent manner. This is the first report demonstrating that topical application of imiquimod can enhance T-cell responses to intracranial tumors as a single agent. The results must be interpreted with caution considering anatomical and biological differences between mice and humans. Nevertheless, Aldara that is being used as a vaccine adjuvant in clinical trials may have direct antitumor effects that are independent of exogenous antigen. Further studies in humans are warranted.
Figures



References
-
- A AG, Tyring SK, Rosen T. Beyond a decade of 5% imiquimod topical therapy. J Drugs Dermatol. 2009;8:467–474. - PubMed
-
- Mohty M, Vialle-Castellano A, Nunes JA, Isnardon D, Olive D, Gaugler B. IFN-alpha skews monocyte differentiation into Toll-like receptor 7-expressing dendritic cells with potent functional activities. J Immunol. 2003;171:3385–3393. - PubMed
-
- Smits EL, Ponsaerts P, Berneman ZN, Van Tendeloo VF. The use of TLR7 and TLR8 ligands for the enhancement of cancer immunotherapy. Oncologist. 2008;13:859–875. - PubMed
-
- Li VW, Li WW, Talcott KE, Zhai AW. Imiquimod as an antiangiogenic agent. J Drugs Dermatol. 2005;4:708–717. - PubMed
-
- Meyer T, Nindl I, Schmook T, Ulrich C, Sterry W, Stockfleth E. Induction of apoptosis by Toll-like receptor-7 agonist in tissue cultures. Br J Dermatol. 2003;149 Suppl 66:9–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

