CYP3A5 genotype does not influence everolimus in vitro metabolism and clinical pharmacokinetics in renal transplant recipients
- PMID: 21389905
- PMCID: PMC3324087
- DOI: 10.1097/TP.0b013e31820ae4ac
CYP3A5 genotype does not influence everolimus in vitro metabolism and clinical pharmacokinetics in renal transplant recipients
Abstract
Background: CYP3A5 genotyping might be useful to guide tacrolimus and sirolimus dosing. The aim of this study was to assess the influence of CYP3A5 polymorphism on everolimus metabolism and pharmacokinetics.
Methods: We investigated the effect of CYP3A5 6986A>G polymorphism (CYP3A5*1/*3 alleles) on the pharmacokinetics of everolimus in 28 renal transplant patients and on its in vitro hepatic metabolism using a bank of genotyped human liver microsomes (n=49). We further evaluated in vitro the contribution of CYP3A4, CYP3A5, and CYP2C8 to everolimus hepatic metabolism using recombinant enzymes.
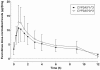
Results: We found no association between CYP3A5 polymorphism and everolimus pharmacokinetics in renal transplant patients. On the other hand, no effect of CYP3A5 polymorphism was observed on the intrinsic clearance of everolimus by human liver microsomes, whereas that of tacrolimus (positive control) was 1.5-fold higher in microsomes carrying the CYP3A5*1 allele than in noncarriers. In vitro data showed that CYP3A4 is a better catalyst of everolimus metabolism than CYP3A5, whereas the opposite was observed for tacrolimus.
Conclusions: This study provides direct and indirect evidence that CYP3A5 genotyping cannot help improve everolimus therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Influence of CYP3A4, CYP3A5 and MDR-1 polymorphisms on tacrolimus pharmacokinetics and early renal dysfunction in liver transplant recipients.Gene. 2013 Jan 10;512(2):226-31. doi: 10.1016/j.gene.2012.10.048. Epub 2012 Oct 26. Gene. 2013. PMID: 23107770
-
Prediction of Tacrolimus Exposure by CYP3A5 Genotype and Exposure of Co-Administered Everolimus in Japanese Renal Transplant Recipients.Int J Mol Sci. 2018 Mar 16;19(3):882. doi: 10.3390/ijms19030882. Int J Mol Sci. 2018. PMID: 29547545 Free PMC article.
-
Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part I.Clin Pharmacokinet. 2010 Mar;49(3):141-75. doi: 10.2165/11317350-000000000-00000. Clin Pharmacokinet. 2010. PMID: 20170205 Review.
-
Effects of CYP3A4 and CYP3A5 polymorphisms on tacrolimus pharmacokinetics in Chinese adult renal transplant recipients: a population pharmacokinetic analysis.Pharmacogenet Genomics. 2013 May;23(5):251-61. doi: 10.1097/FPC.0b013e32835fcbb6. Pharmacogenet Genomics. 2013. PMID: 23459029
-
Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part II.Clin Pharmacokinet. 2010 Apr;49(4):207-21. doi: 10.2165/11317550-000000000-00000. Clin Pharmacokinet. 2010. PMID: 20214406 Review.
Cited by
-
Pharmacogenetics of immunosuppressant drugs: A new aspect for individualized therapy.World J Transplant. 2020 May 29;10(5):90-103. doi: 10.5500/wjt.v10.i5.90. World J Transplant. 2020. PMID: 32864355 Free PMC article. Review.
-
Individualized immunosuppression in transplant patients: potential role of pharmacogenetics.Pharmgenomics Pers Med. 2012;5:63-72. doi: 10.2147/PGPM.S21743. Epub 2012 Jun 18. Pharmgenomics Pers Med. 2012. PMID: 23226063 Free PMC article.
-
The impact of CYP3A5*3 polymorphism on sirolimus pharmacokinetics: insights from predictions with a physiologically-based pharmacokinetic model.Br J Clin Pharmacol. 2015 Dec;80(6):1438-46. doi: 10.1111/bcp.12743. Epub 2015 Oct 28. Br J Clin Pharmacol. 2015. PMID: 26256674 Free PMC article.
-
A Limited Sampling Strategy to Estimate Exposure of Everolimus in Whole Blood and Peripheral Blood Mononuclear Cells in Renal Transplant Recipients Using Population Pharmacokinetic Modeling and Bayesian Estimators.Clin Pharmacokinet. 2018 Nov;57(11):1459-1469. doi: 10.1007/s40262-018-0646-5. Clin Pharmacokinet. 2018. PMID: 29556934
-
Pharmacogenetics in kidney transplantation: recent updates and potential clinical applications.Mol Diagn Ther. 2012 Dec;16(6):331-45. doi: 10.1007/s40291-012-0012-5. Mol Diagn Ther. 2012. PMID: 23192462 Review.
References
-
- Formica RN, Jr, Lorber KM, Friedman AL, et al. The evolving experience using everolimus in clinical transplantation. Transplant Proc. 2004;36 (2 Suppl):495S. - PubMed
-
- Jacobsen W, Serkova N, Hausen B, Morris RE, Benet LZ, Christians U. Comparison of the in vitro metabolism of the macrolide immunosuppressants sirolimus and RAD. Transplant Proc. 2001;33 (1–2):514. - PubMed
-
- Thervet E, Loriot MA, Barbier S, et al. Optimization of Initial Tacrolimus Dose Using Pharmacogenetic Testing. Clin Pharmacol Ther. 2010;87 (6):721. - PubMed
-
- MacPhee IA, Holt DW. A pharmacogenetic strategy for immunosuppression based on the CYP3A5 genotype. Transplantation. 2008;85 (2):163. - PubMed
-
- Anglicheau D, Le Corre D, Lechaton S, et al. Consequences of genetic polymorphisms for sirolimus requirements after renal transplant in patients on primary sirolimus therapy. Am J Transplant. 2005;5 (3):595. - PubMed

