Beyond natural antibodies: the power of in vitro display technologies
- PMID: 21390033
- PMCID: PMC3057417
- DOI: 10.1038/nbt.1791
Beyond natural antibodies: the power of in vitro display technologies
Abstract
In vitro display technologies, best exemplified by phage and yeast display, were first described for the selection of antibodies some 20 years ago. Since then, many antibodies have been selected and improved upon using these methods. Although it is not widely recognized, many of the antibodies derived using in vitro display methods have properties that would be extremely difficult, if not impossible, to obtain by immunizing animals. The first antibodies derived using in vitro display methods are now in the clinic, with many more waiting in the wings. Unlike immunization, in vitro display permits the use of defined selection conditions and provides immediate availability of the sequence encoding the antibody. The amenability of in vitro display to high-throughput applications broadens the prospects for their wider use in basic and applied research.
Figures
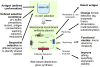


References
-
- Love JC, Ronan JL, Grotenbreg GM, van der Veen AG, Ploegh HL. A microengraving method for rapid selection of single cells producing antigen-specific antibodies. Nat Biotechnol. 2006 - PubMed
-
- Smith GP. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science. 1985;228:1315–1317. - PubMed
-
- Scott JK, Smith GP. Searching for peptide ligands with an epitope library. Science. 1990;249:386–390. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

