The light responsive transcriptome of the zebrafish: function and regulation
- PMID: 21390203
- PMCID: PMC3039656
- DOI: 10.1371/journal.pone.0017080
The light responsive transcriptome of the zebrafish: function and regulation
Abstract
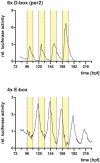
Most organisms possess circadian clocks that are able to anticipate the day/night cycle and are reset or "entrained" by the ambient light. In the zebrafish, many organs and even cultured cell lines are directly light responsive, allowing for direct entrainment of the clock by light. Here, we have characterized light induced gene transcription in the zebrafish at several organizational levels. Larvae, heart organ cultures and cell cultures were exposed to 1- or 3-hour light pulses, and changes in gene expression were compared with controls kept in the dark. We identified 117 light regulated genes, with the majority being induced and some repressed by light. Cluster analysis groups the genes into five major classes that show regulation at all levels of organization or in different subset combinations. The regulated genes cover a variety of functions, and the analysis of gene ontology categories reveals an enrichment of genes involved in circadian rhythms, stress response and DNA repair, consistent with the exposure to visible wavelengths of light priming cells for UV-induced damage repair. Promoter analysis of the induced genes shows an enrichment of various short sequence motifs, including E- and D-box enhancers that have previously been implicated in light regulation of the zebrafish period2 gene. Heterologous reporter constructs with sequences matching these motifs reveal light regulation of D-box elements in both cells and larvae. Morpholino-mediated knock-down studies of two homologues of the D-box binding factor Tef indicate that these are differentially involved in the cell autonomous light induction in a gene-specific manner. These findings suggest that the mechanisms involved in period2 regulation might represent a more general pathway leading to light induced gene expression.
Conflict of interest statement
Figures


 , heart specific genes.
, heart specific genes.



References
-
- Dunlap J, Loros J, DeCoursey P. Sunderland: Sinauer Associates; 2004. Chronobiology.
-
- Dickmeis T. Glucocorticoids and the circadian clock. J Endocrinol. 2009;200:3–22. - PubMed
-
- Vallone D, Lahiri K, Dickmeis T, Foulkes NS. Start the clock! Circadian rhythms and development. Dev Dyn. 2007;236:142–155. - PubMed
-
- Berson DM. Phototransduction in ganglion-cell photoreceptors. Pflugers Arch. 2007;454:849–855. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

