Dynamic conformational changes in munc18 prevent syntaxin binding
- PMID: 21390273
- PMCID: PMC3048386
- DOI: 10.1371/journal.pcbi.1001097
Dynamic conformational changes in munc18 prevent syntaxin binding
Abstract
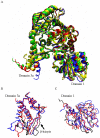
The Sec1/munc18 protein family is essential for vesicle fusion in eukaryotic cells via binding to SNARE proteins. Protein kinase C modulates these interactions by phosphorylating munc18a thereby reducing its affinity to one of the central SNARE members, syntaxin-1a. The established hypothesis is that the reduced affinity of the phosphorylated munc18a to syntaxin-1a is a result of local electrostatic repulsion between the two proteins, which interferes with their compatibility. The current study challenges this paradigm and offers a novel mechanistic explanation by revealing a syntaxin-non-binding conformation of munc18a that is induced by the phosphomimetic mutations. In the present study, using molecular dynamics simulations, we explored the dynamics of the wild-type munc18a versus phosphomimetic mutant munc18a. We focused on the structural changes that occur in the cavity between domains 3a and 1, which serves as the main syntaxin-binding site. The results of the simulations suggest that the free wild-type munc18a exhibits a dynamic equilibrium between several conformations differing in the size of its cavity (the main syntaxin-binding site). The flexibility of the cavity's size might facilitate the binding or unbinding of syntaxin. In silico insertion of phosphomimetic mutations into the munc18a structure induces the formation of a conformation where the syntaxin-binding area is rigid and blocked as a result of interactions between residues located on both sides of the cavity. Therefore, we suggest that the reduced affinity of the phosphomimetic mutant/phosphorylated munc18a is a result of the closed-cavity conformation, which makes syntaxin binding energetically and sterically unfavorable. The current study demonstrates the potential of phosphorylation, an essential biological process, to serve as a driving force for dramatic conformational changes of proteins modulating their affinity to target proteins.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures








References
-
- Hata Y, Slaughter CA, Sudhof TC. Synaptic vesicle fusion complex contains unc-18 homologue bound to syntaxin. Nature. 1993;366:347–351. - PubMed
-
- Gengyo-Ando K, Kamiya Y, Yamakawa A, Kodaira K, Nishiwaki K, et al. The C. elegans unc-18 gene encodes a protein expressed in motor neurons. Neuron. 1993;11:703–711. - PubMed
-
- Voets T, Toonen RF, Brian EC, de Wit H, Moser T, et al. Munc18-1 promotes large dense-core vesicle docking. Neuron. 2001;31:581–591. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

