Critical role of neuropeptides B/W receptor 1 signaling in social behavior and fear memory
- PMID: 21390312
- PMCID: PMC3044739
- DOI: 10.1371/journal.pone.0016972
Critical role of neuropeptides B/W receptor 1 signaling in social behavior and fear memory
Abstract
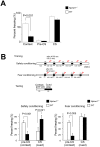
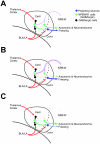
Neuropeptide B/W receptor 1 (NPBWR1) is a G-protein coupled receptor, which was initially reported as an orphan receptor, and whose ligands were identified by this and other groups in 2002 and 2003. To examine the physiological roles of NPBWR1, we examined phenotype of Npbwr1⁻/⁻ mice. When presented with an intruder mouse, Npbwr1⁻/⁻ mice showed impulsive contact with the strange mice, produced more intense approaches toward them, and had longer contact and chasing time along with greater and sustained elevation of heart rate and blood pressure compared to wild type mice. Npbwr1⁻/⁻ mice also showed increased autonomic and neuroendocrine responses to physical stress, suggesting that impairment of NPBWR1 leads to stress vulnerability. We also observed that these mice show abnormality in the contextual fear conditioning test. These data suggest that NPBWR1 plays a critical role in limbic system function and stress responses. Histological and electrophysiological studies showed that NPBWR1 acts as an inhibitory regulator on a subpopulation of GABAergic neurons in the lateral division of the CeA and terminates stress responses. These findings suggest important roles of NPBWR1 in regulating amygdala function during physical and social stress.
Conflict of interest statement
Figures



References
-
- Brezillon S, Lannoy V, Franssen JD, Le Poul E, Dupriez V, et al. Identification of natural ligands for the orphan G protein-coupled receptors GPR7 and GPR8. J Biol Chem. 2003;278:776–783. Epub 2002 Oct 2024. - PubMed
-
- Fujii R, Yoshida H, Fukusumi S, Habata Y, Hosoya M, et al. Identification of a neuropeptide modified with bromine as an endogenous ligand for GPR7. J Biol Chem. 2002;277:34010–34016 Epub 32002 Jul 34012. - PubMed
-
- O'Dowd BF, Scheideler MA, Nguyen T, Cheng R, Rasmussen JS, et al. The cloning and chromosomal mapping of two novel human opioid-somatostatin-like receptor genes, GPR7 and GPR8, expressed in discrete areas of the brain. Genomics. 1995;28:84–91. - PubMed
-
- Hondo M, Ishii M, Sakurai T. The NPB/NPW neuropeptide system and its role in regulating energy homeostasis, pain, and emotion. Results Probl Cell Differ. 2008;46:239–256. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

