Dioxygen activation in soluble methane monooxygenase
- PMID: 21391602
- PMCID: PMC3079780
- DOI: 10.1021/ar1001473
Dioxygen activation in soluble methane monooxygenase
Abstract
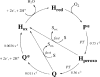
The controlled oxidation of methane to methanol is a chemical transformation of great value, particularly in the pursuit of alternative fuels, but the reaction remains underutilized industrially because of inefficient and costly synthetic procedures. In contrast, methane monooxygenase enzymes (MMOs) from methanotrophic bacteria achieve this chemistry efficiently under ambient conditions. In this Account, we discuss the first observable step in the oxidation of methane at the carboxylate-bridged diiron active site of the soluble MMO (sMMO), namely, the reductive activation of atmospheric O(2). The results provide benchmarks against which the dioxygen activation mechanisms of other bacterial multicomponent monooxygenases can be measured. Molecular oxygen reacts rapidly with the reduced diiron(II) cen-ter of the hydroxylase component of sMMO (MMOH). The first spectroscopically characterized intermediate that results from this process is a peroxodiiron(III) species, P*, in which the iron atoms have identical environments. P* converts to a second peroxodiiron(III) unit, H(peroxo), in a process accompanied by the transfer of a proton, probably with the assistance of a residue near the active site. Proton-promoted O-O bond scission and rearrangement of the diiron core then leads to a diiron(IV) unit, termed Q, that is directly responsible for the oxidation of methane to methanol. In one section of this Account, we provide a detailed discussion of these processes, with particular emphasis on possible structures of the intermediates. The geometries of P* and H(peroxo) are currently unknown, and recent synthetic modeling chemistry has highlighted the need for further structural characterization of Q, currently assigned as a di(μ-oxo)diiron(IV) "diamond core." In another section of the Account, we discuss in detail proton transfer during the O(2) activation events. The role of protons in promoting O-O bond cleavage, thereby initiating the conversion of H(peroxo) to Q, was previously a controversial topic. Recent studies of the mechanism, covering a range of pH values and in D(2)O instead of H(2)O, confirmed conclusively that the transfer of protons, possibly at or near the active site, is necessary for both P*-to-H(peroxo) and H(peroxo)-to-Q conversions. Specific mechanistic insights into these processes are provided. In the final section of the Account, we present our view of experiments that need to be done to further define crucial aspects of sMMO chemistry. Here our goal is to detail the challenges that we and others face in this research, particularly with respect to some long-standing questions about the system, as well as approaches that might be used to solve them.
Figures

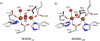




References
-
- Arakawa H, Aresta M, Armor JN, Barteau MA, Beckman EJ, Bell AT, Bercaw JE, Creutz C, Dinjus E, Dixon DA, Domen K, DuBois DL, Eckert J, Fujita E, Gibson DH, Goddard WA, Goodman DW, Keller J, Kubas GJ, Kung HH, Lyons JE, Manzer LE, Marks TJ, Morokuma K, Nicholas KM, Periana R, Que L, Rostrup-Nielson J, Sachtler WMH, Schmidt LD, Sen A, Somorjai GA, Stair PC, Stults BR, Tumas W. Catalysis Research of Relevance to Carbon Management: Progress, Challenges, and Opportunities. Chem. Rev. 2001;101:953–996. - PubMed
-
- Merkx M, Kopp DA, Sazinsky MH, Blazyk JL, Müller J, Lippard SJ. Dioxygen Activation and Methane Hydroxylation by Soluble Methane Monooxygenase: A Tale of Two Irons and Three Proteins. Angew. Chem. Int. Ed. 2001;40:2782–2807. - PubMed
-
- Wallar BJ, Lipscomb JD. Dioxygen Activation by Enzymes Containing Binuclear Non-Heme Iron Clusters. Chem. Rev. 1996;96:2625–2657. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

