Role of metalation in the topoisomerase IIα inhibition and antiproliferation activity of a series of α-heterocyclic-N4-substituted thiosemicarbazones and their Cu(II) complexes
- PMID: 21391686
- PMCID: PMC4151564
- DOI: 10.1021/jm101532u
Role of metalation in the topoisomerase IIα inhibition and antiproliferation activity of a series of α-heterocyclic-N4-substituted thiosemicarbazones and their Cu(II) complexes
Abstract
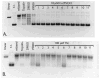
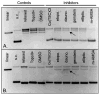
The topoisomerase-IIα inhibition and antiproliferative activity of α-heterocyclic thiosemicarbazones and their corresponding copper(II) complexes have been investigated. The Cu(II)(thiosemicarbazonato)Cl complexes were shown to catalytically inhibit topoisomerase-IIα at concentrations (0.3-7.2 μM) over an order of magnitude lower than their corresponding thiosemicarbazone ligands alone. The copper complexes were also shown to inhibit the proliferation of breast cancer cells expressing high levels of topoisomerase-IIα (SK-BR-3) at lower concentrations than cells expressing lower levels of the enzyme (MCF-7).
Figures
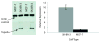



References
-
- Yu Y, Kalinowski DS, Kovacevic Z, Siafakas AR, Jansson PJ, Stefani C, Lovejoy DB, Sharpe PC, Bernhardt PV, Richardson DR. Thiosemicarbazones from the old to new: iron chelators that are more than just ribonucleotide reductase inhibitors. J Med Chem. 2009;52:5271–5294. - PubMed
-
- West DX, Liberta AE. Thiosemicarbazone complexes of copper(II): structural and biological studies. Coord Chem Rev. 1993;123:49–71.
-
- Beraldo H, Gambino D. The wide pharmacological versatility of semicarbazones, thiosemicarbazones and their metal complexes. Mini-Rev Med Chem. 2004;4(1):31–39. - PubMed
-
- Kalinowski DS, Quach P, Richardson DR. Thiosemicarbazones: the new wave in cancer treatment. Future Med Chem. 2009;1(6):1143–1151. - PubMed
-
- Tisato F, Marzano C, Porchia M, Pellei M, Santini C. Copper in diseases and treatments, and copper-based anticancer strategies. Med Res Rev. 2010;30(4):708–749. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information

