Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease
- PMID: 21391816
- PMCID: PMC3294376
- DOI: 10.1146/annurev-biochem-060608-102511
Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease
Abstract
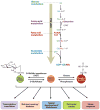
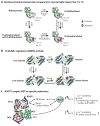
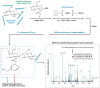
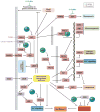
O-GlcNAcylation is the addition of β-D-N-acetylglucosamine to serine or threonine residues of nuclear and cytoplasmic proteins. O-linked N-acetylglucosamine (O-GlcNAc) was not discovered until the early 1980s and still remains difficult to detect and quantify. Nonetheless, O-GlcNAc is highly abundant and cycles on proteins with a timescale similar to protein phosphorylation. O-GlcNAc occurs in organisms ranging from some bacteria to protozoans and metazoans, including plants and nematodes up the evolutionary tree to man. O-GlcNAcylation is mostly on nuclear proteins, but it occurs in all intracellular compartments, including mitochondria. Recent glycomic analyses have shown that O-GlcNAcylation has surprisingly extensive cross talk with phosphorylation, where it serves as a nutrient/stress sensor to modulate signaling, transcription, and cytoskeletal functions. Abnormal amounts of O-GlcNAcylation underlie the etiology of insulin resistance and glucose toxicity in diabetes, and this type of modification plays a direct role in neurodegenerative disease. Many oncogenic proteins and tumor suppressor proteins are also regulated by O-GlcNAcylation. Current data justify extensive efforts toward a better understanding of this invisible, yet abundant, modification. As tools for the study of O-GlcNAc become more facile and available, exponential growth in this area of research will eventually take place.
Figures

References
-
- Torres CR, Hart GW. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J Biol Chem. 1984;259:3308–17. - PubMed
-
- Holt GD, Hart GW. The subcellular distribution of terminal N-acetylglucosamine moieties. Localization of a novel protein-saccharide linkage, O-linked GlcNAc. J Biol Chem. 1986;261:8049–57. - PubMed
-
- Dorfman A, Roseman S, Moses FE, Ludowieg J, Mayeda M. The biosynthesis of hyaluronic acid by group a streptococcus. III. Origin of the N-acetylglucosamine moiety. J Biol Chem. 1955;212:583–91. - PubMed
-
- Marshall S, Bacote V, Traxinger RR. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem. 1991;266:4706–12. - PubMed
-
- Ghosh S, Blumenthal HJ, Davidson E, Roseman S. Glucosamine metabolism. V Enzymatic synthesis of glucosamine 6-phosphate. J Biol Chem. 1960;235:1265–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA042486-28/CA/NCI NIH HHS/United States
- R01 DK061671-10/DK/NIDDK NIH HHS/United States
- R01 CA042486/CA/NCI NIH HHS/United States
- R37 HD013563/HD/NICHD NIH HHS/United States
- R33 DK071280-04/DK/NIDDK NIH HHS/United States
- R33 DK071280/DK/NIDDK NIH HHS/United States
- R01 DK061671/DK/NIDDK NIH HHS/United States
- R37 HD013563-30/HD/NICHD NIH HHS/United States
- R01 CA42486/CA/NCI NIH HHS/United States
- R01 DK61671/DK/NIDDK NIH HHS/United States
- P30 DK079637/DK/NIDDK NIH HHS/United States
- R24 DK084949/DK/NIDDK NIH HHS/United States
- R24 DK084949-01/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

