The phospholipase A1 activity of lysophospholipase A-I links platelet activation to LPA production during blood coagulation
- PMID: 21393252
- PMCID: PMC3073468
- DOI: 10.1194/jlr.M013326
The phospholipase A1 activity of lysophospholipase A-I links platelet activation to LPA production during blood coagulation
Abstract
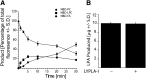
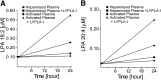
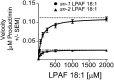
Platelet activation initiates an upsurge in polyunsaturated (18:2 and 20:4) lysophosphatidic acid (LPA) production. The biochemical pathway(s) responsible for LPA production during blood clotting are not yet fully understood. Here we describe the purification of a phospholipase A(1) (PLA(1)) from thrombin-activated human platelets using sequential chromatographic steps followed by fluorophosphonate (FP)-biotin affinity labeling and proteomics characterization that identified acyl-protein thioesterase 1 (APT1), also known as lysophospholipase A-I (LYPLA-I; accession code O75608) as a novel PLA(1). Addition of this recombinant PLA(1) significantly increased the production of sn-2-esterified polyunsaturated LPCs and the corresponding LPAs in plasma. We examined the regioisomeric preference of lysophospholipase D/autotaxin (ATX), which is the subsequent step in LPA production. To prevent acyl migration, ether-linked regioisomers of oleyl-sn-glycero-3-phosphocholine (lyso-PAF) were synthesized. ATX preferred the sn-1 to the sn-2 regioisomer of lyso-PAF. We propose the following LPA production pathway in blood: 1) Activated platelets release PLA(1); 2) PLA(1) generates a pool of sn-2 lysophospholipids; 3) These newly generated sn-2 lysophospholipids undergo acyl migration to yield sn-1 lysophospholipids, which are the preferred substrates of ATX; and 4) ATX cleaves the sn-1 lysophospholipids to generate sn-1 LPA species containing predominantly 18:2 and 20:4 fatty acids.
Figures




References
-
- Mills G. B., Moolenaar W. H. 2003. The emerging role of lysophosphatidic acid in cancer. Nat. Rev. Cancer. 3: 582–591. - PubMed
-
- Tigyi G., Parrill A. L. 2003. Molecular mechanisms of lysophosphatidic acid action. Prog. Lipid Res. 42: 498–526. - PubMed
-
- Pages C., Simon M. F., Valet P., Saulnier-Blache J. S. 2001. Lysophosphatidic acid synthesis and release. Prostaglandins Other Lipid Mediat. 64: 1–10. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

