Pharmacokinetics and pharmacodynamics of intravenous daptomycin during continuous ambulatory peritoneal dialysis
- PMID: 21393490
- PMCID: PMC3087774
- DOI: 10.2215/CJN.08510910
Pharmacokinetics and pharmacodynamics of intravenous daptomycin during continuous ambulatory peritoneal dialysis
Abstract
Background and objectives: This study sought to (1) characterize the pharmacokinetic (PK) profile of intravenous (i.v.) daptomycin among patients receiving continuous ambulatory peritoneal dialysis (CAPD); (2) identify optimal i.v. CAPD dosing schemes; and (3) determine extent of daptomycin penetration into the peritoneal space after i.v. administration.
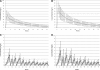
Design, setting, participants, & measurements: A PK study was conducted among eight CAPD patients. Population PK modeling and Monte Carlo simulation (MCS) were used to identify CAPD dosing schemes providing efficacy and toxicity plasma profiles comparable with those obtained from MCS using the daptomycin population PK model derived from patients in the Staphylococcus aureus bacteremia-infective endocarditis (SAB-IE) study. The primary efficacy exposure target was the area under the curve (AUC). For toxicity, the goal was to identify CAPD dosing schemes that minimized plasma trough concentrations in excess of 24.3 mg/L. Finally, peritoneal cavity penetration was determined.
Results: Administration of i.v. daptomycin 4 or 6 mg/kg, depending on indication, every 48 h was identified as the optimal CAPD dosing scheme. This regimen provided cumulative (AUC(0-48)) and daily partitioned (AUC(0-24 h) and AUC(24-48 h)) plasma AUC values similar to the SAB-IE or "typical patient" simulations. In addition, the proportion of patients likely to experience an elevated trough concentration in excess of 24.3 mg/L was similar between every 48 h CAPD dosing and the referent group. Penetration into the peritoneal cavity was 6% of plasma.
Conclusions: Daptomycin 4 or 6 mg/kg, on the basis of indication, i.v. every 48 h was found to be the optimal i.v. CAPD dosing scheme.
Copyright © 2011 by the American Society of Nephrology
Figures

References
-
- Wong SS, Ho PL, Yuen KY: Evolution of antibiotic resistance mechanisms and their relevance to dialysis-related infections. Perit Dial Int 27[Suppl 2]: S272–S280, 2007 - PubMed
-
- Krishnan M, Thodis E, Ikonomopoulos D, Vidgen E, Chu M, Bargman JM, Vas SI, Oreopoulos DG: Predictors of outcome following bacterial peritonitis in peritoneal dialysis. Perit Dial Int 22: 573–581, 2002 - PubMed
-
- Lodise TP, Patel N, Lomaestro BM, Rodvold KA, Drusano GL: Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis 49: 507–514, 2009 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

