IL-21 is pivotal in determining age-dependent effectiveness of immune responses in a mouse model of human hepatitis B
- PMID: 21393863
- PMCID: PMC3049376
- DOI: 10.1172/JCI44198
IL-21 is pivotal in determining age-dependent effectiveness of immune responses in a mouse model of human hepatitis B
Abstract
HBV is a noncytopathic hepadnavirus and major human pathogen that causes immune-mediated acute and chronic hepatitis. The immune response to HBV antigens is age dependent: viral clearance occurs in most adults, while neonates and children usually develop chronic infection and liver disease. Here, we characterize an animal model for HBV infection that recapitulates the key differences in viral clearance between early life and adulthood and find that IL-21 may be part of an effective primary hepatic immune response to HBV. In our model, adult mice showed higher HBV-dependent IL-21 production in liver, compared with that of young mice. Conversely, absence of the IL-21 receptor in adult mice resulted in antigen persistence akin to that of young mice. In humans, levels of IL-21 transcripts were greatly increased in blood samples from acutely infected adults who clear the virus. These observations suggest a different model for the dichotomous, age dependent outcome of HBV infection in humans, in which decreased IL-21 production in younger patients may hinder generation of crucial CD8+ T and B cell responses. These findings carry implications for therapeutic augmentation of immune responses to HBV and potentially other persistent liver viruses.
Figures



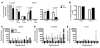
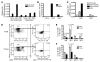


Comment in
-
Viral hepatitis: Interleukin 21 has a key role in age-dependent response to HBV.Nat Rev Gastroenterol Hepatol. 2011 May;8(5):243. doi: 10.1038/nrgastro.2011.36. Epub 2011 Mar 29. Nat Rev Gastroenterol Hepatol. 2011. PMID: 21451485 No abstract available.
-
Antiviral immunity: IL-21 comes with age.Nat Rev Immunol. 2011 Apr;11(4):236-7. doi: 10.1038/nri2964. Nat Rev Immunol. 2011. PMID: 21553713 No abstract available.
-
Hepatitis B virus infection: a strong case against ageism.Hepatology. 2011 Oct;54(4):1477-9. doi: 10.1002/hep.24604. Hepatology. 2011. PMID: 21956709 No abstract available.
References
-
- Robinson WS. Hepatitis B virus and hepatitis D virus. In: Mandell GL, Bennett JE, Dolin R, eds.Principles and Practice of Infectious Diseases . 4th ed. New York, New York, USA: Churchill Livingstone; 1995:1406–1439.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

