Lysosomal positioning coordinates cellular nutrient responses
- PMID: 21394080
- PMCID: PMC3071334
- DOI: 10.1038/ncb2204
Lysosomal positioning coordinates cellular nutrient responses
Abstract
mTOR (mammalian target of rapamycin) signalling and macroautophagy (henceforth autophagy) regulate numerous pathological and physiological processes, including cellular responses to altered nutrient levels. However, the mechanisms regulating mTOR and autophagy remain incompletely understood. Lysosomes are dynamic intracellular organelles intimately involved both in the activation of mTOR complex 1 (mTORC1) signalling and in degrading autophagic substrates. Here we report that lysosomal positioning coordinates anabolic and catabolic responses with changes in nutrient availability by orchestrating early plasma-membrane signalling events, mTORC1 signalling and autophagy. Activation of mTORC1 by nutrients correlates with its presence on peripheral lysosomes that are physically close to the upstream signalling modules, whereas starvation causes perinuclear clustering of lysosomes, driven by changes in intracellular pH. Lysosomal positioning regulates mTORC1 signalling, which in turn influences autophagosome formation. Lysosome positioning also influences autophagosome-lysosome fusion rates, and thus controls autophagic flux by acting at both the initiation and termination stages of the process. Our findings provide a physiological role for the dynamic state of lysosomal positioning in cells as a coordinator of mTORC1 signalling with autophagic flux.
© 2011 Macmillan Publishers Limited. All rights reserved
Figures
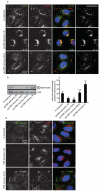
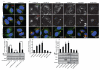
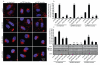
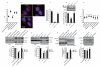
Comment in
-
Lysosome positioning coordinates mTORC1 activity and autophagy.Nat Cell Biol. 2011 Apr;13(4):342-4. doi: 10.1038/ncb0411-342. Nat Cell Biol. 2011. PMID: 21460804
References
-
- Luzio JP, Pryor PR, Bright NA. Lysosomes: fusion and function. Nat Rev Mol Cell Biol. 2007;8:622–632. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

