Cancer radioimmunotherapy
- PMID: 21395378
- PMCID: PMC3123828
- DOI: 10.2217/imt.10.114
Cancer radioimmunotherapy
Abstract
Targeting of radionuclides with antibodies, or radioimmunotherapy, has been an active field of research spanning nearly 50 years, evolving with advancing technologies in molecular biology and chemistry, and with many important preclinical and clinical studies illustrating the benefits, but also the challenges, which all forms of targeted therapies face. There are currently two radiolabeled antibodies approved for the treatment of non-Hodgkin lymphoma, but radioimmunotherapy of solid tumors remains a challenge. Novel antibody constructs, focusing on treatment of localized and minimal disease, and pretargeting are all promising new approaches that are currently under investigation.
Figures

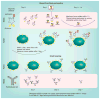
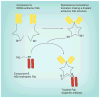
References
-
- Grossbard ML, Press OW, Appelbaum FR, Bernstein ID, Nadler LM. Monoclonal antibody-based therapies of leukemia and lymphoma. Blood. 1992;80(4):863–878. - PubMed
-
- Sharkey RM, Burton J, Goldenberg DM. Radioimmunotherapy of non-Hodgkin’s lymphoma: a critical appraisal. Expert Rev Clin Immunol. 2005;1(1):47–62. - PubMed
-
- Sharkey RM, Goldenberg DM. Perspectives on cancer therapy with radiolabeled monoclonal antibodies. J Nucl Med. 2005;46(Suppl 1):S115–S127. - PubMed
-
- Nelson AL, Dhimolea E, Reichert JM. Development trends for human monoclonal antibody therapeutics. Nat Rev Drug Discov. 2010;9(10):767–774. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
