Single-cell redox imaging demonstrates a distinctive response of dopaminergic neurons to oxidative insults
- PMID: 21395478
- PMCID: PMC3135271
- DOI: 10.1089/ars.2010.3629
Single-cell redox imaging demonstrates a distinctive response of dopaminergic neurons to oxidative insults
Abstract
Aims: The study of the intracellular oxido-reductive (redox) state is of extreme relevance to the dopamine (DA) neurons of the substantia nigra pars compacta. These cells possess a distinct physiology intrinsically associated with elevated reactive oxygen species production, and they selectively degenerate in Parkinson's disease under oxidative stress conditions. To test the hypothesis that these cells display a unique redox response to mild, physiologically relevant oxidative insults when compared with other neuronal populations, we sought to develop a novel method for quantitatively assessing mild variations in intracellular redox state.
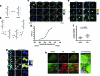
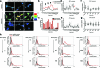
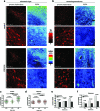
Results: We have developed a new imaging strategy to study redox variations in single cells, which is sensitive enough to detect changes within the physiological range. We studied DA neurons' physiological redox response in biological systems of increasing complexity--from primary cultures to zebrafish larvae, to mammalian brains-and identified a redox response that is distinctive for substantia nigra pars compacta DA neurons. We studied simultaneously, and in the same cells, redox state and signaling activation and found that these phenomena are synchronized.
Innovation: The redox histochemistry method we have developed allows for sensitive quantification of intracellular redox state in situ. As this method is compatible with traditional immunohistochemical techniques, it can be applied to diverse settings to investigate, in theory, any cell type of interest.
Conclusion: Although the technique we have developed is of general interest, these findings provide insights into the biology of DA neurons in health and disease and may have implications for therapeutic intervention.
Figures
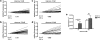

Similar articles
-
Redox sensitivity of tyrosine hydroxylase activity and expression in dopaminergic dysfunction.CNS Neurol Disord Drug Targets. 2012 Jun 1;11(4):419-29. doi: 10.2174/187152712800792938. CNS Neurol Disord Drug Targets. 2012. PMID: 22483306 Review.
-
Dopaminergic neurons.Int J Biochem Cell Biol. 2005 May;37(5):942-6. doi: 10.1016/j.biocel.2004.09.009. Epub 2004 Dec 2. Int J Biochem Cell Biol. 2005. PMID: 15743669 Review.
-
Mitochondria mass is low in mouse substantia nigra dopamine neurons: implications for Parkinson's disease.Exp Neurol. 2007 Feb;203(2):370-80. doi: 10.1016/j.expneurol.2006.08.015. Epub 2006 Sep 28. Exp Neurol. 2007. PMID: 17010972
-
Dopaminergic D2 receptor is a key player in the substantia nigra pars compacta neuronal activation mediated by REM sleep deprivation.Neuropharmacology. 2014 Jan;76 Pt A:118-26. doi: 10.1016/j.neuropharm.2013.08.024. Epub 2013 Sep 4. Neuropharmacology. 2014. PMID: 24012539
-
Ethyl pyruvate rescues nigrostriatal dopaminergic neurons by regulating glial activation in a mouse model of Parkinson's disease.J Immunol. 2011 Jul 15;187(2):960-9. doi: 10.4049/jimmunol.1100009. Epub 2011 Jun 17. J Immunol. 2011. PMID: 21685323
Cited by
-
Characterization of a Novel Monoclonal Antibody against Human Mitochondrial Ferritin and Its Immunohistochemical Application in Human and Monkey Substantia Nigra.Acta Histochem Cytochem. 2017 Feb 28;50(1):49-55. doi: 10.1267/ahc.16034. Epub 2017 Feb 23. Acta Histochem Cytochem. 2017. PMID: 28386150 Free PMC article.
-
Activation of the DNA damage response in vivo in synucleinopathy models of Parkinson's disease.Cell Death Dis. 2018 Jul 26;9(8):818. doi: 10.1038/s41419-018-0848-7. Cell Death Dis. 2018. PMID: 30050065 Free PMC article.
-
Inorganic Polysulfides and Related Reactive Sulfur–Selenium Species from the Perspective of Chemistry.Molecules. 2019 Apr 6;24(7):1359. doi: 10.3390/molecules24071359. Molecules. 2019. PMID: 30959902 Free PMC article. Review.
-
Cholinergic Receptor Modulation as a Target for Preventing Dementia in Parkinson's Disease.Front Neurosci. 2021 Sep 20;15:665820. doi: 10.3389/fnins.2021.665820. eCollection 2021. Front Neurosci. 2021. PMID: 34616271 Free PMC article. Review.
-
Gender biased neuroprotective effect of Transferrin Receptor 2 deletion in multiple models of Parkinson's disease.Cell Death Differ. 2021 May;28(5):1720-1732. doi: 10.1038/s41418-020-00698-4. Epub 2020 Dec 16. Cell Death Differ. 2021. PMID: 33323945 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

