Effect of raltegravir on estradiol and norgestimate plasma pharmacokinetics following oral contraceptive administration in healthy women
- PMID: 21395656
- PMCID: PMC3080652
- DOI: 10.1111/j.1365-2125.2010.03885.x
Effect of raltegravir on estradiol and norgestimate plasma pharmacokinetics following oral contraceptive administration in healthy women
Abstract
Aims: Oral contraceptives such as norgestimate-ethinyl estradiol (Ortho Tri-Cyclen®) are commonly prescribed in the HIV-infected patient population. A placebo-controlled, randomized, two-period crossover study in healthy HIV-seronegative subjects was conducted to assess the effect of raltegravir on the pharmacokinetics of the estrogen and progestin components of norgestimate-ethinyl estradiol [ethinyl estradiol (EE) and norelgestromin (NGMN), an active metabolite of norgestimate (NGT)].
Methods: In each of two periods, nineteen healthy women established on norgestimate-ethinyl estradiol contraception (21 days of active contraception; 7 days of placebo) received either 400 mg raltegravir or matching placebo twice daily on days 1-21. Pharmacokinetics were analysed on day 21 of each period.
Results: The geometric mean ratio (GMR) and 90% confidence interval (CI) for the EE component of norgestimate-ethinyl estradiol when co-administrated with raltegravir relative to EE alone was 0.98 (0.93-1.04) for the area under the concentration-time curve from 0 to 24 h (AUC(0-24 h) ) and 1.06 (0.98-1.14) for the maximum concentration of drug in the plasma (C(max) ); the GMR (90% CI) for the NGMN component of norgestimate-ethinyl estradiol when co-administered with raltegravir relative to NGMN alone was 1.14 (1.08-1.21) for AUC(0-24 h) and 1.29 (1.23-1.37) for C(max) . There were no discontinuations due to a study drug-related adverse experience, nor any serious clinical or laboratory adverse experience.
Conclusions: Raltegravir has no clinically important effect on EE or NGMN pharmacokinetics. Co-administration of raltegravir and an oral contraceptive containing EE and NGT was generally well tolerated; no dose adjustment is required for oral contraceptives containing EE and NGT when co-administered with raltegravir.
© 2011 The Authors. British Journal of Clinical Pharmacology © 2011 The British Pharmacological Society.
Figures
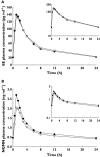
 ); Ortho Tri-Cyclen® + PBO (
); Ortho Tri-Cyclen® + PBO ( )
)References
-
- Merck and Co. I. Isentress package insert. 2008.
-
- Curtis KM, Chrisman CE, Peterson HB. Contraception for women in selected circumstances. Obstet Gynecol. 2002;99:1100–12. - PubMed
-
- Hirai S, Hussain A, Haddadin M, Smith RB. First-pass metabolism of ethinyl estradiol in dogs and rats. J Pharm Sci. 1981;70:403–6. - PubMed
-
- Newburger J, Castracane VD, Moore PH, Jr, Williams MC, Goldzieher JW. The pharmacokinetics and metabolism of ethinyl estradiol and its three sulfates in the baboon. Am J Obstet Gynecol. 1983;146:80–7. - PubMed
-
- Hammond GL, Abrams LS, Creasy GW, Natarajan J, Allen JG, Siiteri PK. Serum distribution of the major metabolites of norgestimate in relation to its pharmacological properties. Contraception. 2003;67:93–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

