Prevalence and distribution of regional scar in dysfunctional myocardial segments in Duchenne muscular dystrophy
- PMID: 21396105
- PMCID: PMC3075215
- DOI: 10.1186/1532-429X-13-20
Prevalence and distribution of regional scar in dysfunctional myocardial segments in Duchenne muscular dystrophy
Abstract
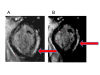
Background: The segmental relationship between cardiovascular magnetic resonance (CMR) peak circumferential strain (Ecc) and myocardial scar has not been well characterized in Duchenne muscular dystrophy (DMD), and it is unknown whether echocardiography accurately measures Ecc in DMD. We assessed segmental Ecc and scar using CMR with myocardial tissue tagging and late gadolinium enhancement (LGE) in patients with DMD, then compared CMR with echocardiographic velocity vector imaging (VVI) for regional Ecc based on independent observer assessments.
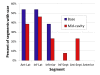
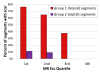
Results: Participants enrolled (n = 16; age 8-23) had median left ventricular (LV) ejection fraction of 0.52 (range 0.28-0.69), and 156 basal and mid-cavity myocardial segments from the 13 patients completing the LGE protocol were analyzed for strain and scar. Segmental CMR Ecc in the most negative quartile (quartile 4) ruled out scar in that segment, but scar was present in 46% of segments in the least negative (most dysfunctional) Ecc quartile 1, 33% of Ecc quartile 2 segments, and 15% of Ecc quartile 3 segments. Overall scar prevalence in inferior, inferolateral, and anterolateral segments was eight times higher than in inferoseptal, anteroseptal, and anterior segments (p < 0.001). This increased proportion of scar in lateral versus septal segments was consistent across CMR Ecc quartiles (quartile 1: 76% versus 11%, p = 0.001; quartile 2: 65% versus 9%, p < 0.001; quartile 3: 38% versus 0%, p < 0.001). Echocardiographic analysis could be performed in 12 of 14 patients with CMR exams and had to be limited to mid-cavity slices. Echo segmental Ecc in the most negative quartile made scar by CMR in that segment highly unlikely, but the correlation in segmental Ecc between CMR and echo was limited (r = 0.27; p = 0.02).
Conclusions: The relationship between scar and Ecc in DMD is complex. Among myocardial segments with depressed Ecc, scar prevalence was much higher in inferior, inferolateral, and anterolateral segments, indicating a regionally dependent association between abnormal Ecc and scar, with free wall segments commonly developing dysfunction with scar and septal segments developing dysfunction without scar. Although normal echocardiographic Ecc predicted absence of scar, regional echocardiographic Ecc by VVI has only a limited association with CMR Ecc in DMD.
Figures


Similar articles
-
Myocardial Strain Using Cardiac MR Feature Tracking and Speckle Tracking Echocardiography in Duchenne Muscular Dystrophy Patients.Pediatr Cardiol. 2018 Mar;39(3):478-483. doi: 10.1007/s00246-017-1777-4. Epub 2017 Nov 29. Pediatr Cardiol. 2018. PMID: 29188318 Free PMC article.
-
Prevalence of scarred and dysfunctional myocardium in patients with heart failure of ischaemic origin: a cardiovascular magnetic resonance study.J Cardiovasc Magn Reson. 2011 Sep 21;13(1):53. doi: 10.1186/1532-429X-13-53. J Cardiovasc Magn Reson. 2011. PMID: 21936915 Free PMC article.
-
Presence of mechanical dyssynchrony in Duchenne muscular dystrophy.J Cardiovasc Magn Reson. 2011 Feb 2;13(1):12. doi: 10.1186/1532-429X-13-12. J Cardiovasc Magn Reson. 2011. PMID: 21288342 Free PMC article.
-
T1-Mapping and extracellular volume estimates in pediatric subjects with Duchenne muscular dystrophy and healthy controls at 3T.J Cardiovasc Magn Reson. 2020 Dec 10;22(1):85. doi: 10.1186/s12968-020-00687-z. J Cardiovasc Magn Reson. 2020. PMID: 33302967 Free PMC article.
-
Prevalence and distribution of late gadolinium enhancement in a large population of patients with Duchenne muscular dystrophy: effect of age and left ventricular systolic function.J Cardiovasc Magn Reson. 2013 Dec 21;15(1):107. doi: 10.1186/1532-429X-15-107. J Cardiovasc Magn Reson. 2013. PMID: 24359596 Free PMC article.
Cited by
-
Mapping contrast agent uptake and retention in MRI studies of myocardial perfusion: case control study of dogs with Duchenne muscular dystrophy.Int J Cardiovasc Imaging. 2013 Apr;29(4):819-26. doi: 10.1007/s10554-012-0137-y. Epub 2012 Oct 17. Int J Cardiovasc Imaging. 2013. PMID: 23070737 Free PMC article.
-
The Use of Speckle Tracking Echocardiography for Early Detection of Myocardial Dysfunction in Patients with Duchenne Muscular Dystrophy.Pediatr Cardiol. 2016 Dec;37(8):1422-1428. doi: 10.1007/s00246-016-1451-2. Epub 2016 Jul 25. Pediatr Cardiol. 2016. PMID: 27452803
-
Stabilization of Early Duchenne Cardiomyopathy With Aldosterone Inhibition: Results of the Multicenter AIDMD Trial.J Am Heart Assoc. 2019 Oct;8(19):e013501. doi: 10.1161/JAHA.119.013501. Epub 2019 Sep 24. J Am Heart Assoc. 2019. PMID: 31549577 Free PMC article. Clinical Trial.
-
Natural History of Histopathologic Changes in Cardiomyopathy of Golden Retriever Muscular Dystrophy.Front Vet Sci. 2022 Feb 17;8:759585. doi: 10.3389/fvets.2021.759585. eCollection 2021. Front Vet Sci. 2022. PMID: 35252412 Free PMC article.
-
Myocardial Strain Using Cardiac MR Feature Tracking and Speckle Tracking Echocardiography in Duchenne Muscular Dystrophy Patients.Pediatr Cardiol. 2018 Mar;39(3):478-483. doi: 10.1007/s00246-017-1777-4. Epub 2017 Nov 29. Pediatr Cardiol. 2018. PMID: 29188318 Free PMC article.
References
-
- Gilroy J, Calahan JL, Berman R, Newman M. Cardiac and pulmonary complications in Duchenne's progressive muscular dystrophy. Circulation. 1963;27:484–493. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

