Purification and biochemical properties of a cytochrome bc complex from the aerobic hyperthermophilic archaeon Aeropyrum pernix
- PMID: 21396131
- PMCID: PMC3062577
- DOI: 10.1186/1471-2180-11-52
Purification and biochemical properties of a cytochrome bc complex from the aerobic hyperthermophilic archaeon Aeropyrum pernix
Abstract
Background: The bioenergetics of Archaea with respect to the evolution of electron transfer systems is very interesting. In contrast to terminal oxidases, a canonical bc1 complex has not yet been isolated from Archaea. In particular, c-type cytochromes have been reported only for a limited number of species.
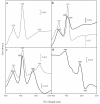
Results: Here, we isolated a c-type cytochrome-containing enzyme complex from the membranes of the hyperthermophilic archaeon, Aeropyrum pernix, grown aerobically. The redox spectrum of the isolated c-type cytochrome showed a characteristic α-band peak at 553 nm corresponding to heme C. The pyridine hemochrome spectrum also revealed the presence of heme B. In non-denaturing polyacrylamide gel electrophoresis, the cytochrome migrated as a single band with an apparent molecular mass of 80 kDa, and successive SDS-PAGE separated the 80-kDa band into 3 polypeptides with apparent molecular masses of 40, 30, and 25 kDa. The results of mass spectrometry indicated that the 25-kDa band corresponded to the hypothetical cytochrome c subunit encoded by the ORF APE_1719.1. In addition, the c-type cytochrome-containing polypeptide complex exhibited menaquinone: yeast cytochrome c oxidoreductase activities.
Conclusion: In conclusion, we showed that A. pernix, a hyperthemophilic archaeon, has a "full" bc complex that includes a c-type cytochrome, and to the best of our knowledge, A. pernix is the first archaea from which such a bc complex has been identified. However, an electron donor candidates for cytochrome c oxidase, such as a blue copper protein, have not yet been identified in the whole genome data of this archaeon. We are currently trying to identify an authentic substrate between a bc complex and terminal oxidase.
Figures




References
-
- Sakamoto J, Sone N. In: Respiration in archaea and bacteria. Zannoni D, editor. Vol. 1. The Netherlands: Kluwer Academic Publishers; 2004. Biochemical and Molecular Features of Terminal Oxidases; pp. 87–113.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

