CNNM2, encoding a basolateral protein required for renal Mg2+ handling, is mutated in dominant hypomagnesemia
- PMID: 21397062
- PMCID: PMC3059432
- DOI: 10.1016/j.ajhg.2011.02.005
CNNM2, encoding a basolateral protein required for renal Mg2+ handling, is mutated in dominant hypomagnesemia
Abstract
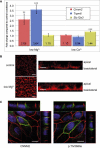
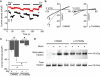
Familial hypomagnesemia is a rare human disorder caused by renal or intestinal magnesium (Mg(2+)) wasting, which may lead to symptoms of Mg(2+) depletion such as tetany, seizures, and cardiac arrhythmias. Our knowledge of the physiology of Mg(2+) (re)absorption, particularly the luminal uptake of Mg(2+) along the nephron, has benefitted from positional cloning approaches in families with Mg(2+) reabsorption disorders; however, basolateral Mg(2+) transport and its regulation are still poorly understood. Here, by using a candidate screening approach, we identified CNNM2 as a gene involved in renal Mg(2+) handling in patients of two unrelated families with unexplained dominant hypomagnesemia. In the kidney, CNNM2 was predominantly found along the basolateral membrane of distal tubular segments involved in Mg(2+) reabsorption. The basolateral localization of endogenous and recombinant CNNM2 was confirmed in epithelial kidney cell lines. Electrophysiological analysis showed that CNNM2 mediated Mg(2+)-sensitive Na(+) currents that were significantly diminished in mutant protein and were blocked by increased extracellular Mg(2+) concentrations. Our data support the findings of a recent genome-wide association study showing the CNNM2 locus to be associated with serum Mg(2+) concentrations. The mutations found in CNNM2, its observed sensitivity to extracellular Mg(2+), and its basolateral localization signify a critical role for CNNM2 in epithelial Mg(2+) transport.
Copyright © 2011 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures
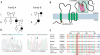

References
-
- Quamme G.A. Renal magnesium handling: New insights in understanding old problems. Kidney Int. 1997;52:1180–1195. - PubMed
-
- Simon D.B., Lu Y., Choate K.A., Velazquez H., Al-Sabban E., Praga M., Casari G., Bettinelli A., Colussi G., Rodriguez-Soriano J. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science. 1999;285:103–106. - PubMed
-
- Konrad M., Schaller A., Seelow D., Pandey A.V., Waldegger S., Lesslauer A., Vitzthum H., Suzuki Y., Luk J.M., Becker C. Mutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement. Am. J. Hum. Genet. 2006;79:949–957. - PMC - PubMed
-
- Hou J., Paul D.L., Goodenough D.A. Paracellin-1 and the modulation of ion selectivity of tight junctions. J. Cell Sci. 2005;118:5109–5118. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

