Chemical shift tensors: theory and application to molecular structural problems
- PMID: 21397119
- PMCID: PMC3058154
- DOI: 10.1016/j.pnmrs.2010.10.003
Chemical shift tensors: theory and application to molecular structural problems
Figures
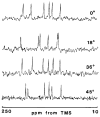
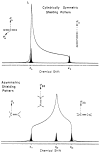
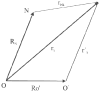
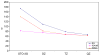

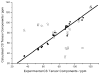

References
-
- Purcell EM. A precise determination of the proton magnetic moment in Bohr magnetons. Phys Rev. 76(1949):1262–1263.
-
- Purcell EM, Torrey HC, Pound RV. Resonance absorption by nuclear magnetic moments in a solid. Phys Rev. 1946;69:37–38.
-
- Ramsey NF. Magnetic shielding of nuclei in molecules. Phys Rev. 1950;78:699–703.
-
- Ramsey NF. The internal diamagnetic field correction in measurements of the proton magnetic moment. Phys Rev. 1950;77:567.
-
- Jameson CJ, de Dios AC. Theoretical and physical aspects of nuclear shielding, Nuclear Magnetic Resonance an Specialist Periodical Reports. The Royal Chemical Society; London: 2009. pp. 68–93.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

