Lower peripheral blood CD14+ monocyte frequency and higher CD34+ progenitor cell frequency are associated with HBV vaccine induced response in HIV infected individuals
- PMID: 21397720
- PMCID: PMC3081920
- DOI: 10.1016/j.vaccine.2011.02.092
Lower peripheral blood CD14+ monocyte frequency and higher CD34+ progenitor cell frequency are associated with HBV vaccine induced response in HIV infected individuals
Abstract
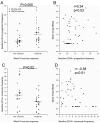
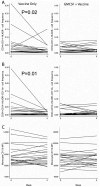
We evaluated immunologic predictors of response to HBV vaccine administered in the presence or absence of GM-CSF in HIV infected individuals. We measured peripheral blood hematopoietic progenitor, monocyte and myeloid-derived suppressor cell (MDSC) frequencies, and expression of GMCSF receptor on monocytes and MDSCs, at baseline and 4weeks after immunization in relation to antibody response. We observed higher baseline progenitor and lower monocyte frequencies among week 16 antibody responders. Week 4 decline in MDSC frequency was associated with week 16 antibody response, while administration of GM-CSF was associated with preservation of these cells. No significant differences in GM-CSF receptor expression were observed in the presence vs. absence of GM-CSF. These findings are consistent with a positive role of progenitor cells and a potential negative role of monocytes in vaccine response. Additionally, GM-CSF augmented the preservation of peripheral blood MDSC, which may contribute to the lack of improved vaccine responses.
Trial registration: ClinicalTrials.gov NCT00272493.
Published by Elsevier Ltd.
Figures

Similar articles
-
Immune response to hepatitis B vaccine in HIV-infected subjects using granulocyte-macrophage colony-stimulating factor (GM-CSF) as a vaccine adjuvant: ACTG study 5220.Vaccine. 2010 Aug 2;28(34):5597-604. doi: 10.1016/j.vaccine.2010.06.030. Epub 2010 Jun 23. Vaccine. 2010. PMID: 20600512 Free PMC article. Clinical Trial.
-
Dendritic cells generated from CD34+ progenitor cells with flt3 ligand, c-kit ligand, GM-CSF, IL-4, and TNF-alpha are functional antigen-presenting cells resembling mature monocyte-derived dendritic cells.J Immunother. 2000 Jan;23(1):48-58. doi: 10.1097/00002371-200001000-00007. J Immunother. 2000. PMID: 10687137
-
Peripheral blood dendritic cells and GM-CSF as an adjuvant for hepatitis B vaccination in hemodialysis patients.Kidney Int. 2004 Aug;66(2):614-21. doi: 10.1111/j.1523-1755.2004.00781.x. Kidney Int. 2004. PMID: 15253714 Clinical Trial.
-
Efficacy of granulocyte-macrophage colony-stimulating factor (GM-CSF) as a vaccine adjuvant for hepatitis B virus in patients with HIV infection.Vaccine. 2003 Nov 7;21(31):4545-9. doi: 10.1016/s0264-410x(03)00500-0. Vaccine. 2003. PMID: 14575766 Clinical Trial.
-
Hepatitis B virus vaccine in lymphoproliferative disorders: a prospective randomized study evaluating the efficacy of granulocyte-macrophage colony stimulating factor as a vaccine adjuvant.Eur J Haematol. 2007 Oct;79(4):292-6. doi: 10.1111/j.1600-0609.2007.00912.x. Epub 2007 Jul 26. Eur J Haematol. 2007. PMID: 17655695 Clinical Trial.
Cited by
-
Improved CD4 T cell profile in HIV-infected subjects on maraviroc-containing therapy is associated with better responsiveness to HBV vaccination.J Transl Med. 2018 Aug 29;16(1):238. doi: 10.1186/s12967-018-1617-1. J Transl Med. 2018. PMID: 30157873 Free PMC article.
-
Hepatitis B virus large surface protein: function and fame.Hepatobiliary Surg Nutr. 2015 Feb;4(1):1-10. doi: 10.3978/j.issn.2304-3881.2014.12.08. Hepatobiliary Surg Nutr. 2015. PMID: 25713800 Free PMC article. Review.
-
The Role of Immune Cells in Chronic HBV Infection.J Clin Transl Hepatol. 2015 Dec 28;3(4):277-83. doi: 10.14218/JCTH.2015.00026. Epub 2015 Dec 15. J Clin Transl Hepatol. 2015. PMID: 26807384 Free PMC article. Review.
-
Long noncoding RNA HOTAIRM1 promotes myeloid-derived suppressor cell expansion and suppressive functions through up-regulating HOXA1 expression during latent HIV infection.AIDS. 2020 Dec 1;34(15):2211-2221. doi: 10.1097/QAD.0000000000002700. AIDS. 2020. PMID: 33048872 Free PMC article.
-
Significant roles of regulatory T cells and myeloid derived suppressor cells in hepatitis B virus persistent infection and hepatitis B virus-related HCCs.Int J Mol Sci. 2015 Feb 3;16(2):3307-22. doi: 10.3390/ijms16023307. Int J Mol Sci. 2015. PMID: 25654227 Free PMC article. Review.
References
-
- Hilleman MR. Overview of the pathogenesis, prophylaxis and therapeusis of viral hepatitis B, with focus on reduction to practical applications. Vaccine. 2001 Feb 28;19(15-16):1837–48. - PubMed
-
- Ada G. Overview of vaccines. Mol Biotechnol. 1997 Oct;8(2):123–34. - PubMed
-
- Hess G, Clemens R, Bienzle U, Schonfeld C, Schunck B, Bock HL. Immunogenicity and safety of an inactivated hepatitis A vaccine in anti-HIV positive and negative homosexual men. J Med Virol. 1995 May;46(1):40–2. - PubMed
-
- Kemper CA, Haubrich R, Frank I, Dubin G, Buscarino C, McCutchan JA, et al. Safety and immunogenicity of hepatitis A vaccine in human immunodeficiency virus-infected patients: a double-blind, randomized, placebo-controlled trial. J Infect Dis. 2003 Apr 15;187(8):1327–31. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U01 AI069502/AI/NIAID NIH HHS/United States
- UM1 AI069501/AI/NIAID NIH HHS/United States
- AI038855/AI/NIAID NIH HHS/United States
- AI0801/AI/NIAID NIH HHS/United States
- R01 DK068361/DK/NIDDK NIH HHS/United States
- U01 AI069532/AI/NIAID NIH HHS/United States
- AI069495/AI/NIAID NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- U01 AI069495/AI/NIAID NIH HHS/United States
- AI 68634/AI/NIAID NIH HHS/United States
- UM1 AI069532/AI/NIAID NIH HHS/United States
- AI 68636/AI/NIAID NIH HHS/United States
- U01 AI025879/AI/NIAID NIH HHS/United States
- AI069532/AI/NIAID NIH HHS/United States
- AI 068634/AI/NIAID NIH HHS/United States
- UM1 AI069495/AI/NIAID NIH HHS/United States
- U01 AI038855/AI/NIAID NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- U01 AI069501/AI/NIAID NIH HHS/United States
- U01 AI068634/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

