Variability in protist grazing and growth on different marine Synechococcus isolates
- PMID: 21398485
- PMCID: PMC3126417
- DOI: 10.1128/AEM.02241-10
Variability in protist grazing and growth on different marine Synechococcus isolates
Abstract
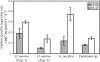
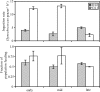
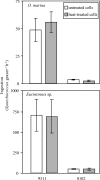
Grazing mortality of the marine phytoplankton Synechococcus is dominated by planktonic protists, yet rates of consumption and factors regulating grazer-Synechococcus interactions are poorly understood. One aspect of predator-prey interactions for which little is known are the mechanisms by which Synechococcus avoids or resists predation and, in turn, how this relates to the ability of Synechococcus to support growth of protist grazer populations. Grazing experiments conducted with the raptorial dinoflagellate Oxyrrhis marina and phylogenetically diverse Synechococcus isolates (strains WH8102, CC9605, CC9311, and CC9902) revealed marked differences in grazing rates-specifically that WH8102 was grazed at significantly lower rates than all other isolates. Additional experiments using the heterotrophic nanoflagellate Goniomonas pacifica and the filter-feeding tintinnid ciliate Eutintinnis sp. revealed that this pattern in grazing susceptibility among the isolates transcended feeding guilds and grazer taxon. Synechococcus cell size, elemental ratios, and motility were not able to explain differences in grazing rates, indicating that other features play a primary role in grazing resistance. Growth of heterotrophic protists was poorly coupled to prey ingestion and was influenced by the strain of Synechococcus being consumed. Although Synechococcus was generally a poor-quality food source, it tended to support higher growth and survival of G. pacifica and O. marina relative to Eutintinnis sp., indicating that suitability of Synechococcus varies among grazer taxa and may be a more suitable food source for the smaller protist grazers. This work has developed tractable model systems for further studies of grazer-Synechococcus interactions in marine microbial food webs.
Figures



References
-
- Allali K., Dolan J., Rassoulzadegan F. 1994. Culture characteristics and orthophosphate excretion of a marine oligotrich ciliate, Strombidium-Sulcatum, fed heat-killed bacteria. Mar. Ecol. Prog. Ser. 105:159–165
-
- Baudoux A. C., Veldhuis M. J. W., Noordeloos A. A. M., van Noort G., Brussaard C. P. D. 2008. Estimates of virus- vs. grazing-induced mortality of picophytoplankton in the North Sea during summer. Aquat. Microb. Ecol. 52:69–82
-
- Boenigk J., Arndt H. 2000. Particle handling during interception feeding by four species of heterotrophic nanoflagellates. J. Eukaryot. Microbiol. 47:350–358 - PubMed
-
- Boenigk J., Matz C., Jurgens K., Arndt H. 2001. Confusing selective feeding with differential digestion in bacterivorous nanoflagellates. J. Eukaryot. Microbiol. 48:425–432 - PubMed
-
- Boenigk J., Matz C., Jürgens K., Arndt H. 2001. The influence of preculture conditions and food quality on the ingestion and digestion process of three species of heterotrophic nanoflagellates. Microb. Ecol. 42:168–176 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

