C-peptide, a novel inhibitor of lung inflammation following hemorrhagic shock
- PMID: 21398498
- PMCID: PMC3094028
- DOI: 10.1152/ajplung.00308.2010
C-peptide, a novel inhibitor of lung inflammation following hemorrhagic shock
Abstract
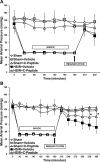
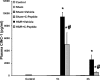
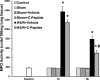
C-peptide is a 31-amino acid peptide cleaved from proinsulin during insulin synthesis. Initially thought to be inert, C-peptide may modulate the inflammatory response in the setting of endotoxemia and ischemia reperfusion. However, the spectrum of its biological effects is unclear. We hypothesized that exogenous administration of C-peptide would modulate pro- and anti-inflammatory signaling pathways and thereby attenuate lung inflammation in an in vivo model of hemorrhagic shock. Hemorrhagic shock was induced in male Wistar rats (aged 3-4 mo) by withdrawing blood to a mean arterial pressure of 50 mmHg. At 3 h after hemorrhage, rats were rapidly resuscitated by returning their shed blood. At the time of resuscitation and every hour thereafter, animals received C-peptide (280 nmol/kg) or vehicle parenterally. Animals were euthanized at 1 and 3 h after resuscitation. C-peptide administration at resuscitation following hemorrhagic shock ameliorated hypotension and blunted the systemic inflammatory response by reducing plasma levels of IL-1, IL-6, macrophage inflammatory protein-1α, and cytokine-induced neutrophil chemoattractant-1. This was associated with a reduction in lung neutrophil infiltration and plasma levels of receptor for advanced glycation end products. Mechanistically, C-peptide treatment was associated with reduced expression of proinflammatory transcription factors activator protein-1 and NF-κB and activation of the anti-inflammatory transcription factor peroxisome proliferator-activated receptor-γ. Our data suggest that C-peptide ameliorates the inflammatory response and lung inflammation following hemorrhagic shock. These effects may be modulated by altering the balance between pro- and anti-inflammatory signaling in the lung.
Figures







References
-
- Abraham E. Neutrophils and acute lung injury. Crit Care Med 31: S195–S199, 2003 - PubMed
-
- Abraham E. NF-kappaB activation. Crit Care Med 28: N100–N104, 2000 - PubMed
-
- Adams M, Reginato MJ, Shao D, Lazar MA, Chatterjee VK. Transcriptional activation by peroxisome proliferator-activated receptor gamma is inhibited by phosphorylation at a consensus mitogen-activated protein kinase site. J Biol Chem 272: 5128–5132, 1997 - PubMed
-
- Al-Rasheed NM, Chana RS, Baines RJ, Willars GB, Brunskill NJ. Ligand-independent activation of peroxisome proliferator-activated receptor-gamma by insulin and C-peptide in kidney proximal tubular cells: dependent on phosphatidylinositol 3-kinase activity. J Biol Chem 279: 49747–49754, 2004 - PubMed
-
- Al-Rasheed NM, Meakin F, Royal EL, Lewington AJ, Brown J, Willars GB, Brunskill NJ. Potent activation of multiple signalling pathways by C-peptide in opossum kidney proximal tubular cells. Diabetologia 47: 987–997, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

