Arrhythmogenic substrate in hearts of rats with monocrotaline-induced pulmonary hypertension and right ventricular hypertrophy
- PMID: 21398591
- PMCID: PMC3119089
- DOI: 10.1152/ajpheart.01226.2010
Arrhythmogenic substrate in hearts of rats with monocrotaline-induced pulmonary hypertension and right ventricular hypertrophy
Abstract
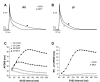
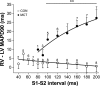
Mechanisms associated with right ventricular (RV) hypertension and arrhythmias are less understood than those in the left ventricle (LV). The aim of our study was to investigate whether and by what mechanisms a proarrhythmic substrate exists in a rat model of RV hypertension and hypertrophy. Rats were injected with monocrotaline (MCT; 60 mg/kg) to induce pulmonary artery hypertension or with saline (CON). Myocardial levels of mRNA for genes expressing ion channels were measured by real-time RT-PCR. Monophasic action potential duration (MAPD) was recorded in isolated Langendorff-perfused hearts. MAPD restitution was measured, and arrhythmias were induced by burst stimulation. Twenty-two to twenty-six days after treatment, MCT animals had RV hypertension, hypertrophy, and decreased ejection fractions compared with CON. A greater proportion of MCT hearts developed sustained ventricular tachycardias/fibrillation (0.83 MCT vs. 0.14 CON). MAPD was prolonged in RV and less so in the LV of MCT hearts. There were decreased levels of mRNA for K(+) channels. Restitution curves of MCT RV were steeper than CON RV or either LV. Dispersion of MAPD was greater in MCT hearts and was dependent on stimulation frequency. Computer simulations based on ion channel gene expression closely predicted experimental changes in MAPD and restitution. We have identified a proarrhythmic substrate in the hearts of MCT-treated rats. We conclude that steeper RV electrical restitution and rate-dependant RV-LV action potential duration dispersion may be contributing mechanisms and be implicated in the generation of arrhythmias associated with in RV hypertension and hypertrophy.
Figures



References
-
- Basso C, Corrado D, Marcus FI, Nava A, Thiene G. Arrhythmogenic right ventricular cardiomyopathy. Lancet 373: 1289–1300, 2009 - PubMed
-
- Bogaard HJ, Abe K, Vonk NA, Voelkel NF. The right ventricle under pressure: cellular and molecular mechanisms of right-heart failure in pulmonary hypertension. Chest 135: 794–804, 2009 - PubMed
-
- Buermans HP, Redout EM, Schiel AE, Musters RJ, Zuidwijk M, Eijk PP, van HC, Kasanmoentalib S, Visser FC, Ylstra B, Simonides WS. Microarray analysis reveals pivotal divergent mRNA expression profiles early in the development of either compensated ventricular hypertrophy or heart failure. Physiol Genomics 21: 314–323, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

