Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS
- PMID: 21398619
- PMCID: PMC3107729
- DOI: 10.1093/jnci/djr027
Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS
Abstract
Background: Ipsilateral breast tumor recurrence (IBTR) is the most common failure event after lumpectomy for ductal carcinoma in situ (DCIS). We evaluated invasive IBTR (I-IBTR) and its influence on survival among participants in two National Surgical Adjuvant Breast and Bowel Project (NSABP) randomized trials for DCIS.
Methods: In the NSABP B-17 trial (accrual period: October 1, 1985, to December 31, 1990), patients with localized DCIS were randomly assigned to the lumpectomy only (LO, n = 403) group or to the lumpectomy followed by radiotherapy (LRT, n = 410) group. In the NSABP B-24 double-blinded, placebo-controlled trial (accrual period: May 9, 1991, to April 13, 1994), all accrued patients were randomly assigned to LRT+ placebo, (n=900) or LRT + tamoxifen (LRT + TAM, n = 899). Endpoints included I-IBTR, DCIS-IBTR, contralateral breast cancers (CBC), overall and breast cancer-specific survival, and survival after I-IBTR. Median follow-up was 207 months for the B-17 trial (N = 813 patients) and 163 months for the B-24 trial (N = 1799 patients).
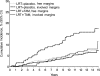
Results: Of 490 IBTR events, 263 (53.7%) were invasive. Radiation reduced I-IBTR by 52% in the LRT group compared with LO (B-17, hazard ratio [HR] of risk of I-IBTR = 0.48, 95% confidence interval [CI] = 0.33 to 0.69, P < .001). LRT + TAM reduced I-IBTR by 32% compared with LRT + placebo (B-24, HR of risk of I-IBTR = 0.68, 95% CI = 0.49 to 0.95, P = .025). The 15-year cumulative incidence of I-IBTR was 19.4% for LO, 8.9% for LRT (B-17), 10.0% for LRT + placebo (B-24), and 8.5% for LRT + TAM. The 15-year cumulative incidence of all contralateral breast cancers was 10.3% for LO, 10.2% for LRT (B-17), 10.8% for LRT + placebo (B-24), and 7.3% for LRT + TAM. I-IBTR was associated with increased mortality risk (HR of death = 1.75, 95% CI = 1.45 to 2.96, P < .001), whereas recurrence of DCIS was not. Twenty-two of 39 deaths after I-IBTR were attributed to breast cancer. Among all patients (with or without I-IBTR), the 15-year cumulative incidence of breast cancer death was 3.1% for LO, 4.7% for LRT (B-17), 2.7% for LRT + placebo (B-24), and 2.3% for LRT + TAM.
Conclusions: Although I-IBTR increased the risk for breast cancer-related death, radiation therapy and tamoxifen reduced I-IBTR, and long-term prognosis remained excellent after breast-conserving surgery for DCIS.
Figures





Comment in
-
Re: Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS.J Natl Cancer Inst. 2011 Nov 16;103(22):1723. doi: 10.1093/jnci/djr406. Epub 2011 Oct 3. J Natl Cancer Inst. 2011. PMID: 21969339 No abstract available.
References
-
- Li CI, Daling JR, Malone KE. Age-specific incidence rates of in situ breast carcinomas by histologic type, 1980 to 2001. Cancer Epidemiol Biomarkers Prev. 2005;14(4):1008–1011. - PubMed
-
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66. - PubMed
-
- Veronessi U, Sacozzi R, del Vecchio M, et al. Comparing radical mastectomy with quadrantectomy, axillary dissection, and radiotherapy in patients with small cancers of the breast. N Engl J Med. 1981;305(1):6–11. - PubMed
-
- Fisher B, Bauer M, Margolese R, et al. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med. 1985;312(11):665–673. - PubMed
-
- Betsill WL Jr, Rosen PP, Lieberman PH, Robbins GF. Intraductal. carcinoma. Long term follow-up after treatment by biopsy alone. JAMA. 1978;239(18):1863–1867. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

