Leptin in immuno-rheumatological diseases
- PMID: 21399656
- PMCID: PMC4012876
- DOI: 10.1038/cmi.2010.75
Leptin in immuno-rheumatological diseases
Abstract
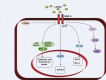
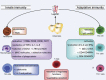
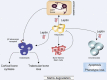
Leptin is one of the most important hormones secreted by adipocytes, with a variety of physiological roles related to the control of metabolism and energy homeostasis. Since its discovery in 1994, leptin has attracted increasing interest in the scientific community for its pleiotropic actions. One of these functions is the relationship between nutritional status and immune competence. It structurally resembles proinflammatory cytokines, such as IL-6 and IL-12. The cytokine-like structural characteristic of leptin is implicative of its function in regulating immune responses. The role of leptin in regulating immune responses has been assessed in vitro as well as in clinical studies. It has been shown that disease conditions of reduced leptin production are associated with increased infection susceptibility. Conversely, immune-mediated disorders, such as autoimmune diseases, are associated with the increased secretion of leptin and the production of proinflammatory pathogenic cytokines. In this paper, we review the most recent advances of the role of leptin in immune-rheumatological diseases, and we discuss whether strategies aimed at modifying leptin levels could represent innovative and therapeutic tools for autoimmune disorders.
Figures
References
-
- Kennedy GC. The role of depot fat in the hypothalamic control of food intake in the rat. Proc R Soc Lond B Biol Sci. 1953;140:578–596. - PubMed
-
- Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J Hered. 1950;41:317–318. - PubMed
-
- Coleman DL. Effects of parabiosis of obese with diabetes and normal mice. Diabetologia. 1973;9:294–298. - PubMed
-
- Ahima RS, Qi Y, Singhal NS, Jackson MB, Scherer PE. Brain adipocytokine action and metabolic regulation. Diabetes. 2006;55:145–154. - PubMed
-
- Lago F, Dieguez C, Gomez-Reino J, Gualillo O. The emerging role of adipokines as mediators of inflammation and immune response. Cytokine Growth Factor Rev. 2007;18:313–325. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

