Ribonucleases endowed with specific toxicity for spermatogenic layers
- PMID: 21399757
- PMCID: PMC3055560
- DOI: 10.1016/S0305-0491(97)00278-2
Ribonucleases endowed with specific toxicity for spermatogenic layers
Abstract
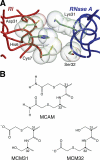
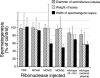
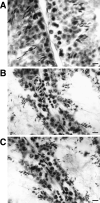
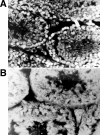
Bovine seminal ribonuclease (BS-RNase) is a dimer in which the subunits are cross-linked by disulfide bonds between Cys31 of one subunit and Cys32 of the other. Dimeric BS-RNase is resistant to ribonuclease inhibitor (RI), a protein endogenous to mammalian cells, and is toxic to a variety of cell types. Monomeric BS-RNase (like its homolog, RNase A) is bound tightly by RI and is not cytotoxic. The three-dimensional structure of the RI·RNase A complex suggests that carboxymethylation of C32S BS-RNase (to give MCM31) or C31S BS-RNase (MCM32) could diminish affinity for RI. We find that MCM31 and MCM32 are not only resistant to RI, but are also aspermatogenic to mice. In contrast to the aspermatogenic activity of dimeric BS-RNase, that of MCM31 and MCM32 is directed only at spermatogenic layers. Intratesticular injection of MCM31 or MCM32 affects neither the diameter of seminiferous tubules nor the weight of testes. Also in contrast to wild-type BS-RNase, MCM31 and MCM32 are not toxic to other cell types. Direct immunofluorescence reveals that MCM31 and MCM32 bind only to spermatogonia and primary spermatocytes. This cell specificity makes MCM31 and MCM32 of potential use in seminoma therapy and contraception.
Figures


References
-
- Ardelt W, Mikulski SM, Shogen K. Amino acid sequence of an anti-tumor protein from Rana pipiens oocytes and early embryos. J. Biol. Chem. 1991;266:245–251. - PubMed
-
- Bartholeyns J, Zenebergh A. In vitro and in vivo antitumor effect of dimerized ribonuclease A. Eur. J. Cancer. 1979;15:85–91. - PubMed
-
- Beintema JJ, Schüller C, Irie M, Carsana A. Molecular evolution of the ribonuclease superfamily. Prog. Biophys. Molec. Biol. 1988;51:165–192. - PubMed
-
- Blackburn P, Gavilanes JG. Identification of lysine residues in the binding domain of ribonuclease A for the RNase inhibitor from human placenta. J. Biol. Chem. 1982;255:10959–10965. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
