Interaction between Toll-like receptors and natural killer cells in the destruction of bile ducts in primary biliary cirrhosis
- PMID: 21400555
- PMCID: PMC3077894
- DOI: 10.1002/hep.24194
Interaction between Toll-like receptors and natural killer cells in the destruction of bile ducts in primary biliary cirrhosis
Abstract
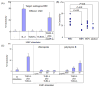
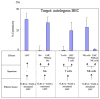
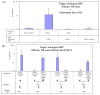
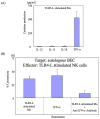
Primary biliary cirrhosis (PBC) is characterized by chronic nonsuppurative destructive cholangitis (CNSDC) associated with destruction of small bile ducts. Although there have been significant advances in the dissection of the adaptive immune response against the mitochondrial autoantigens, there are increasing data that suggest a contribution of innate immune mechanisms in inducing chronic biliary pathology. We have taken advantage of our ability to isolate subpopulations of liver mononuclear cells (LMC) and examined herein the role of Toll-like receptors (TLRs), their ligands, and natural killer (NK) cells in modulating cytotoxic activity against biliary epithelial cells (BECs). In particular, we demonstrate that Toll-like receptor 4 ligand (TLR4-L)-stimulated NK cells destroy autologous BECs in the presence of interferon alpha (IFN-α) synthesized by TLR 3 ligand (TLR3-L)-stimulated monocytes (Mo). Indeed, IFN-α production by hepatic Mo is significantly increased in patients with PBC compared to disease controls. There were also marked increases in the cytotoxic activity of hepatic NK cells from PBC patients compared to NK cells from controls but only when the NK cells were prepared following ligation of both TLR3-L- and TLR4-L-stimulated LMC. These functional data are supported by the immunohistochemical observation of an increased presence of CD56-positive NK cells scattered around destroyed small bile ducts more frequently in liver tissues from PBC patients than controls.
Conclusion: These data highlight critical differences in the varied roles of Mo and NK cells following TLR3-L and TLR4-L stimulation.
2011 American Association for the Study of Liver Diseases.
Figures

Comment in
-
Natural killer cells take two tolls to destruct bile ducts.Hepatology. 2011 Apr;53(4):1076-9. doi: 10.1002/hep.24275. Hepatology. 2011. PMID: 21384404 Free PMC article. No abstract available.
References
-
- Gershwin ME, Ansari AA, Mackay IR, Nakanuma Y, Nishio A, Rowley MJ, Coppel RL. Primary biliary cirrhosis: an orchestrated immune response against epithelial cells. Immunol Rev. 2000;174:210–225. - PubMed
-
- Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261–1273. - PubMed
-
- McNally RJ, Ducker S, James OF. Are transient environmental agents involved in the cause of primary biliary cirrhosis? Evidence from space-time clustering analysis. Hepatology. 2009;50:1169–1174. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
